- Systematic Review
- Open access
- Published:
Sarcopenia and sarcopenic obesity among older adults in the nordic countries: a scoping review
BMC Geriatrics volume 24, Article number: 421 (2024)
Abstract
Background
Sarcopenia and sarcopenic obesity (SO) are age-related syndromes that may compromise physical and mental health among older adults. The Nordic countries differ from other regions on prevalence of disease, life-style behavior, and life expectancy, which may impact prevalence of sarcopenia and SO. Therefore, the aim of this study is to review the available evidence and gaps within this field in the Nordic countries.
Methods
PubMed, Embase, and Web of science (WOS) were searched up to February 2023. In addition, grey literature and reference lists of included studies were searched. Two independent researcher assessed papers and extracted data.
Results
Thirty-three studies out of 6,363 searched studies were included in this scoping review. Overall prevalence of sarcopenia varied from 0.9 to 58.5%. A wide prevalence range was still present for community-dwelling older adults when definition criteria and setting were considered. The prevalence of SO ranged from 4 to 11%, according to the only study on this field. Based on the included studies, potential risk factors for sarcopenia include malnutrition, low physical activity, specific diseases (e.g., diabetes), inflammation, polypharmacy, and aging, whereas increased levels of physical activity and improved dietary intake may reduce the risk of sarcopenia. The few available interventions for sarcopenia were mainly focused on resistance training with/without nutritional supplements (e.g., protein, vitamin D).
Conclusion
The findings of our study revealed inadequate research on SO but an increasing trend in the number of studies on sarcopenia. However, most of the included studies had descriptive cross-sectional design, small sample size, and applied different diagnostic criteria. Therefore, larger well-designed cohort studies that adhere to uniform recent guidelines are required to capture a full picture of these two age-related medical conditions in Nordic countries, and plan for prevention/treatment accordingly.
Background
The number of older adults with age-related disorders is expected to increase worldwide [1, 2]. Sarcopenia and sarcopenic obesity (SO) are both age-related syndromes that may compromise the physical and mental health of older adults and increase their need for health care services in old age [3, 4], and this may challenge the sustainability of health care systems economically and by shortage of health care personnel [5].
Sarcopenia is characterized by low muscle mass in combination with low muscle strength [4]. SO is characterized by the co-existence of obesity (excessive adipose tissue) and sarcopenia [3]. Sarcopenia and SO are both associated with physical disability, risk of falls, morbidity, reduced quality of life and early mortality [4, 6,7,8,9]. In SO the consequences of sarcopenia and obesity are combined and maximized [4, 6,7,8].
Etiology of sarcopenia and SO is multifactorial and closely linked to multimorbidity [3, 7,8,9,10]. Nevertheless, lifestyle and behavioral components particularly diet and physical activity, are important interrelated factors that potentially can be modified. Physical inactivity and sedentary behavior may accelerate age-related loss of muscle mass, reduce energy expenditure, and increase risk of obesity [3, 11]. In addition, weight cycling (the fluctuations in weight following dieting and regain) and an unbalanced diet (particularly inadequate protein intake) may accelerate loss of muscle mass and increase severity of sarcopenia and SO in older adults [3, 12]. International guideline for the treatment of sarcopenia emphasizes the importance of resistance training potentially in combination with nutritional supplementation to improve muscle mass and physical function [13]. Similar therapeutic approach is suggested for treatment of SO [14]. However, more research is needed to confirm optimal treatment of SO [14].
According to a recently published meta-analysis the global prevalence of sarcopenia ranged from 10 to 27% in populations of older adults ≥ 60 years [15]. Further the global prevalence of SO among older adults was 11% [8]. So, sarcopenia and SO are prevalent conditions, with multiple negative health outcomes and should be given special attention [16]. Despite the large burden on patients and health care systems, the awareness of the importance of skeletal muscle maintenance in obesity is low among clinicians and scientists [3, 16].
A recent meta-analysis on publication trends revealed that despite an increase in global research on sarcopenia, the Nordic countries were only limitedly represented [6]. Nordic countries may differ from other regions on aspects associated with the prevalence and trajectory of sarcopenia and SO and challenge the representativeness of research findings from other parts of the world. These include a different prevalence pattern of noncommunicable diseases [17], different life-style behavior and life-style associated risk factors [15, 18], and higher life expectancy [18].
The Nordic countries including Sweden, Finland, Iceland, Norway, Denmark, and three autonomous areas (Åland Islands, Greenland and Faroe Islands) share common elements of social and economic policies such as a comprehensive publicly financed health care system [18, 19]. Additionally, these countries have a strong tradition of collaboration including a common vision of a socially sustainable region by promoting equal health and inclusive participation in society for older adults [20]. Therefore, more insight into the etiology, prevalence, and risk factors for sarcopenia and SO among older adults is a prerequisite for the development and implementation of effective strategies to prevent and treat these complex geriatric conditions in this geographic region. So, the aim of this study is to conduct a scoping review to systematically identify and map the available evidence while also addressing knowledge gaps and exploring the following research questions: (1) What are the prevalence of sarcopenia and SO in older adults living in the Nordic countries? (2) Which risk factors or contributing conditions are involved in the development of sarcopenia and SO in the Nordic Countries? (3) Which interventions to prevent or counteract negative health outcomes of sarcopenia and SO have been tested or implemented among older adults living in the Nordic countries?
Methods
Identification of relevant studies
The development and reporting of this review were done by following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [21].
The literature search was developed to target three main areas: Sarcopenia, sarcopenic obesity, and aging (See Appendix 1 for full search strategy). All studies published before the end of February 2023 were included in this scoping review. The optimal sensitivity of search was obtained by simultaneous search of the following databases: PubMed, Embase, and Web of science (WOS). Additionally, a detailed search for grey literature was performed in relevant databases (e.g., Research Portal Denmark, Libris, Oria, Research.fi). Besides, reference lists of the included studies were reviewed to identify eligible studies. Duplicates and non-peer reviewed evidence (e.g., PhD thesis) were excluded but if the latter contained published articles of relevance, these were included. If more than one publication on similar outcomes (e.g., prevalence) were based on a single study, just one publication was included. Data were extracted from large studies with combined data from several countries only when findings were presented separately for the Nordic countries.
Inclusion and exclusion criteria
The inclusion criteria were as follow: Broad selection criteria were used to be comprehensive: (1) studies with any outcome (e.g., prevalence, risk factors, etc.) to address our research questions on sarcopenia and SO, (2) studies on subjects with age ≥ 60 years in any type of settings (e.g., community, nursing homes, general practice, hospital, outpatients, homecare, etc.), (3) studies using any definition of sarcopenia and SO without restriction for criteria and cutoff values, (4) all type of study designs (e.g., randomized control trials, cohort studies, cross-sectional, etc.), (5) studies should be conducted in the Nordic countries The exclusion criteria are as follow: (1) studies without relevant outcome to sarcopenia or SO, (2) studies without sufficient information to determine eligibility.
Study selection and data extraction
Two independent researchers screened literature and conducted data extraction. Any discrepancies between them were resolved through discussion.
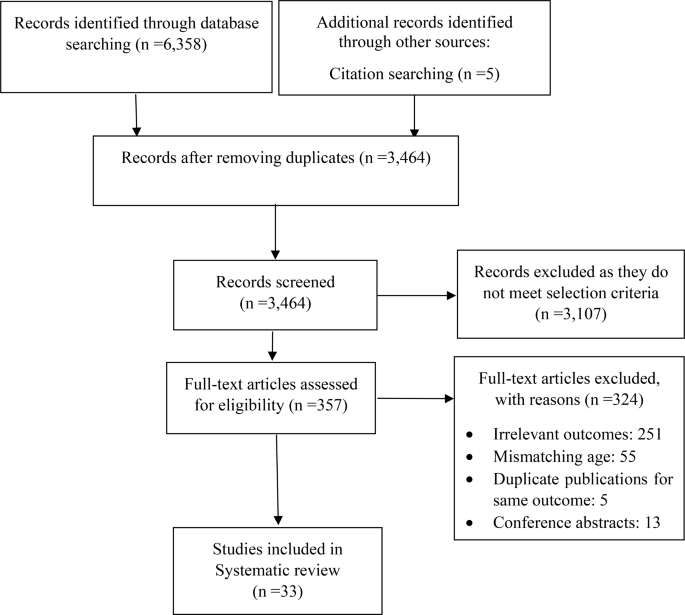
First, duplicates were removed by using EndNote 20.6 software, then titles and abstracts were screened to narrow down the list of potentially eligible studies. Finally, the full text review was done to examine in detail the studies that were not excluded in first step. For more clarification, the reasons for the exclusion were recorded (Fig. 1).
The following information was extracted: (1) study characteristics (e.g., first author’s name, country, year of publication), (2) characteristics of the target population (e.g., age, sex), (3) study design, setting, intervention duration and follow-up time (if applicable), measurements, tools, criteria, and results.
Results
Study selection
A combined total of 6,358 studies were identified through the initial electronic database and grey literature searches. An additional five articles were identified through other sources (citation searching). After removing duplication, 3,464 articles remained. A total of 3107 articles were excluded based on screening titles and abstracts. Out of the remaining 357 studies, 324 were excluded after the full-text review. Finally, 33 studies met our inclusion criteria and were included in this current scoping review [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54] (Fig. 1).
Study characteristics
Table 1 summarized characteristics of the included studies.
The number of documents showed an increasing trend between 2020 and 2021. A peak in the number of publications was observed in 2021 (24.2% of all documents). All the studies were conducted across four (Denmark, Norway, Sweden, and Finland) out of the five Nordic countries and three autonomous areas. The highest contribution in this field was made by Sweden (n = 12).
Most studies were conducted in community-dwelling settings [22,23,24, 28, 30, 31, 35, 36, 38,39,40, 42, 45,46,47,48,49, 54]. Seven studies included patients with acute diseases (hospital-setting) [26, 27, 33, 37, 50,51,52], while four studies included patients with chronic conditions (out-patient setting) [25, 32, 41, 44], and one study including nursing-home residents [34]. In terms of study design, most of the studies were observation studies with a cross-sectional or longitudinal design (22,23,24,25,26,27,28,29,30,31, 33, 34, 36,37,38,39,40,41,42,43,44,45, 46,47,48,49,50,51,52,53,54), while three studies [32, 35, 46] applied interventions. It appears, however, that one study [32] out of the above three interventions is sub-project conducted within the framework of larger intervention program. Sample size ranged from 49 in a cross-sectional case control study [52] to 3334 in a cohort study [30].
Five studies were among males only [22, 24, 36, 45, 53] and three studies included females only [38, 47, 54]. The rest of the studies had a mixed sample. Top subject area was sarcopenia (31 out of the 33 included studies), and on this subject, publications were categorized into the following research areas (with some studies addressing more areas): prevalence [22,23,24,25,26,27, 29, 30, 33, 35, 36, 37, 40, 42, 44, 45, 47, 49,50,51, 52,53,54], risk factors [24, 27, 28, 30, 31, 34, 38, 40, 42, 44, 47, 49,50,51], and effectiveness of interventions on sarcopenia or indicator of sarcopenia [32, 35, 46].
In most studies sarcopenia was defined according to the criteria set by the European Working Group on Sarcopenia in Older People in the updated version from 2019 (EWGSOP2) (n = 15) or the original version from 2010 (EWGSOP) (n = 14). However, in some studies multiple criteria such as EWGSOP, EWGSOP2, and National Institutes of Health Sarcopenia Project definition (FNIH) were applied [27, 39, 43], and in other studies alternative criteria were used [26, 33, 35, 45, 57].
Different assessment methods of muscle mass including Dual energy X-ray absorptiometry (DXA) [22, 24, 25, 27, 29, 30, 32, 33, 38,39,40,41, 45,46,47, 52,53,54], Bioelectrical Impedance Analysis (BIA) [28, 31, 34, 44, 48, 49], Bioimpedance Spectroscopy (BIS) [35, 42, 43], Computed Tomography (CT) [33], and Computed Tomography Angiogram (CTA) [26] were used in the included studies.
SO were defined by the co-existence of sarcopenia with obesity. Studies on SO used the EWGSOP2 criteria [39], or the EWGSOP2 criteria for hand grip strength only (probable sarcopenia) [23] in combination with obesity estimated from BMI cut points [23, 39], waist circumference [23, 39], and fat mass percentage [39]. Lastly, one study used measures of body composition measures that reflect adiposity as estimates of SO [48].
Four studies reported the prevalence of “probable sarcopenia” [23, 30, 36, 45], while two studies reported the prevalence of sarcopenia and comorbidities (e.g., osteopenia, pre-frailty, malnutrition) [33, 40].
Narrative synthesis
Due to the heterogeneity of the studies in definition of sarcopenia, settings, and sample size, the overall reported prevalence was variable and ranged from 0.9% [54] to 58.5% [26]. However, according to the most commonly used criteria (EWGSOP2) the highest (46%) and lowest (1%) prevalence of sarcopenia was reported in Sweden among inpatients in geriatric care [27], and community-dwelling older adults [30], respectively.
Prevalence of sarcopenia according to population and definition criteria is illustrated in Table 2. Higher prevalence rates of sarcopenia were found in females compared to males among community-dwelling older adults [49] and in older adults acutely admitted to hospital [51]. Further, acutely admitted female patients also presented with more severe sarcopenia compared to male patients [51].
Frequency of sarcopenia was higher (9.1–40.0%) in patients with diabetes (with and without complications of charcot osteoarthropathy), compared to age-matched healthy adults [52].
The prevalence of “probable sarcopenia” ranged between 20.4% (reduced muscle strength only) and 38.1% (fulfilling one of the following criteria: reduced muscle strength, reduced muscle mass, or low physical function) in Finnish community-dwelling adults [23, 36], while longitudinal studies on Swedish community-dwelling old (70 years) and very old adults (≥ 85 years) the prevalence of “probable sarcopenia” (reduced muscle strength only) ranged from 1.8 to 73%, respectively [30, 45]. Lastly, in a Swedish study among nursing home residents the prevalence of probable sarcopenia was 44% (evaluated by an impaired chair stand test) [34].
Prevalence of Osteosarcopenia (sarcopenia and osteoporosis) was 1.5% [36], and the prevalence of co-occurrence of all three following conditions: pre-frail, malnutrition, and sarcopenia was 7% [34].
We only identified two studies with prevalence of SO [39] and probable SO [23]. The prevalence of SO in a Swedish population was 4% and 11% in females and males, respectively, while the prevalence of probable SO among Finnish community-dwelling ranged between 5.8% and 12.6%, depending on the criteria to define the obesity (e.g., BMI, waist circumference, etc.) [23].
Several studies investigated aspects of etiology and risk factors for sarcopenia [24, 27, 28, 30, 31, 34, 36, 38, 40, 42,43,44, 47, 49,50,51] and one study focused on SO [49]. Higher physical activity was associated with a decreased likelihood of sarcopenia [30]. In addition, adhering to world health organization (WHO) guidlines for physical activity and the Nordic nutritional recommendations for protein intake was positively associated with greater physical function and lower fat mass in older female community-dwellers [38]. In older adults who are physically active, eating a healthy diet (based on the frequency of intake of favorable food like fish, fruits, vegetables, and whole grains versus unfavorable foods like red/processed meats, desserts/sweets/sugar-sweetened beverages, and fried potatoes) was associated with lower risk of sarcopenia [28]. Further, among older adults who already meet the physical activity guidelines, additional engagement in muscle-strengthening activities was associated with a lower sarcopenia risk score and improved muscle mass and chair rise time [31].
Associations between sarcopenia, risk of sarcopenia and malnutrition or nutritional status was identified in geriatric patients [27, 51], older patients with hip fracture [50], nursing home residents [34] and in community-dwelling older adults [49]. Moreover, the importance of nutritional intake was investigated in the following studies [24, 36, 47]. A study among community-dwelling men revealed an inverse association between total energy intake, protein intake (total, plant, and fish protein), intake of dietary fibers, fat (total and unsaturated), and vitamin D with sarcopenia status [36]. In a cohort of 71-year-old men a dietary pattern characterized by high consumption of fruit, vegetables, poultry, rice and pasta was associated with lower prevalence of sarcopenia after 16 years [24]. A longitudinal Finnish study on sarcopenia indices among postmenopausal older women, showed that lower adherence to the Mediterranean (focuses on high consumption of olive oil) or Baltic Sea (focuses on the dietary fat quality and low-fat milk intake) diets resulted in higher loss of lean mass over a 3-year period [47]. Further, a higher adherence to the Baltic Sea diet was associated with greater lean mass and better physical function, and higher adherence to the Mediterranean diet was associated with greater muscle quality [47].
In a study of patients with hip fracture age, polypharmacy, and low albumin levels was associated with sarcopenia [50]. Exocrine pancreatic insufficiency was an independent risk factor for sarcopenia [44]. This study also revealed that sarcopenia was associated with reduced quality of life, physical function, and increased risk of hospitalization [44]. In a longitudinal study of community-dwelling adults (+ 75 years) at risk of sarcopenia, high physical function, muscle strength, muscle mass and low BMI predicted better physical function and reduced need for care after four years [42]. Furthermore, in community-dwelling adults with sarcopenia, muscle mass, muscle strength and physical function are independent predictors of all-cause mortality. As a result, they have been proposed by researchers as targets for the prevention of sarcopenia-related over-mortality [43]. Lastly, community-dwelling older adults with sarcopenia had lower bone mineral density compared to those without sarcopenia and they were more likely to develop osteoporosis (Osteosarcopenia) [40].
Regarding SO risk factors, a longitudinal study among community-dwelling older adults in Finland found that SO (operationalized by measures of adiposity) were associated with poorer physical function after ten years [48].
Our literature search identified three randomized controlled trials investigating the effectiveness of interventions to prevent or counteract sarcopenia in older adults of Norway, Finland, and Sweden, respectively [32, 35, 46]. The Norwegian study [32] was a double-blinded randomized controlled trial (RCT). The study included those who were at risk of developing sarcopenia, including patients with chronic obstructive pulmonary disease (COPD) or individuals who showed diagnostic indications of sarcopenia. Participants received either vitamin D3 or placebo supplementation for 28 weeks. Additionally, resistance training sessions were provided to all participants from weeks 14 to 27. Vitamin D supplementation did not significantly affect response to resistance training in older adults at risk of sarcopenia with or without COPD [32].
Furthermore, a RCT among pre-sarcopenic Swedish older adults investigated the effectiveness of three weekly sessions of instructor-led progressive resistance training in combination with a non-mandatory daily nutritional supplement (175 kcal, 19 g protein) compared to control group. The 10 weeks intervention resulted in significant between group improvements of physical function and a significant improvement in body composition in the intervention group [46].
Another intervention study revealed that a 12-month intervention with two daily nutritional supplements (each containing 20 g whey protein) did not attenuate the deterioration of physical function and muscle mass in sarcopenic older community-dwelling adults compared to isocaloric placebo supplements or no supplementation. All participants were given instructions on home-based exercises, importance of dietary protein and vitamin D supplementation [35].
Discussion
Based on our broad literature search 33 studies were identified that concerned sarcopenia and SO and met the inclusion criteria. However, research on SO was very limited with only three studies identified. Narrative synthesis of the included studies revealed that the most reported classification tool for sarcopenia in Nordic countries was the EWGSOP2. Moreover, some studies estimated sarcopenia using EWGSOP. The overall prevalence of sarcopenia in Nordic countries according to EWGSOP2 ranged between 1% and 46% [25, 28]. The prevalence of SO, however, was reported only in one study in Sweden (4–11%) [39]. Even though the previous systematic reviews and meta-analysis have reported the prevalence of sarcopenia and SO in different regions and settings (e.g., community-dwelling, nursing home, etc.) [8, 15, 55, 56], this current scoping review is to the best of our knowledge the first study that provides an overview of research on sarcopenia and SO in the Nordic countries.
Based on our findings from 24 studies, there were large variability in prevalence of sarcopenia in studies conducted in the Nordic countries. We think that the wide variation in estimated prevalence of sarcopenia in our scoping review might be due to a different definition/diagnostic criterion (e.g., EWGSOP, EWGSOP2, FNIH), methodology to measure muscle mass (DXA, BIA, CT), and heterogeneity in characteristics of the study population (e.g., setting, age, medical conditions, co-occurrence of multiple risk factors). A previous study on prevalence of sarcopenia in Swedish older people showed significant differences between prevalence of sarcopenia based on EWGSOP2 and EWGSOP1 [29]. Therefore, researchers stressed that prevalence is more dependent on cut-offs than on the operational definition [29, 57]. Further, we know that various international sarcopenia working groups have issued expert consensus and such diagnostic criteria are being updated [4, 58]. Since the revision of criteria focuses primarily on the adjustment of cut-off values, the main reason for differences in prevalence even when using an updated version of one diagnosis criteria is modification in cut-off values. For instance, if the cut-off value for gait speed was increased by 0.2 m/s, the prevalence of sarcopenia may increase by 8.5% [57]. Meaning that even a small change in cut-off value can have a big impact on how sarcopenia is diagnosed. Besides when we take definition criteria into account (Table 2), the prevalence of sarcopenia is still variable in the population of community-dwelling adults for instance. We believe it is basically because studies have applied different assessment tools and tests to identify older adults with low muscle mass and muscle strength, although using the same definition criteria (Table 1). Previous studies have illustrated that choice of methodology to assess muscle strength (e.g., hand grip strength, chair rise) [59] and muscle mass (e.g., DXA, BIA, anthropometry) [60,61,62] in older adults may impact findings and this variability may explain some of the variability in our findings. So, adherence to the latest uniform diagnostic criteria for future studies is recommended to simplify the comparison of findings within the same country, across countries, and regions. Moreover, we suggest that medical community particularly GPs to come to an agreement on assessment methods for muscle mass and muscle strength and the use of one set of definition criteria for sarcopenia.
In previous meta-analyses [15], sub-group analyses based on region and classification tool, revealed that the prevalence of sarcopenia was higher in European studies using EWGSOP (12%) compared to rest of the studies using Asian Working Group for Sarcopenia (AWGS), FNIH, and EWGSOP (3%) [15]. In our scoping review, we also found a high prevalence of sarcopenia in Nordic countries. Longevity and life expectancy is higher in the Nordic countries compared to estimates for rest of the world [18], which means that in this region many people reach old age, and consequently they are more likely to be diagnosed with sarcopenia as an age-related disorder. Therefore, the authors of this current scoping review emphasis the importance of preventive strategies targeted major risk factors and effective interventions to limit the consequences of sarcopenia in the Nordic populations. Besides, we think that the health care system in the Nordic countries should be better equipped with the necessary healthcare resources for both a timely diagnosis and dealing with this major age-related issue in the years to come. However, due to the limitations regarding the timely diagnosis, we highly recommend a comprehensive approach including establishment of support services, implement educational programs, offer training for health care professionals, and engage the community.
Many countries have conducted research on SO [7, 39, 63,64,65]. Based on our findings, however, among the Nordic countries only Sweden and Finland have investigated the prevalence of probable SO and SO [23, 29]. Besides, we only found one study investigating the association between body adiposity and physical function over time [54]. We did not find any literature on risk factors or interventions among older adults with SO in this region. Therefore, we call on medical and research community in Nordic countries to attach importance to screening of SO in elderly people to capture a full picture of this public health risk to aging society and allocate healthcare resources accordingly.
In terms of risk factors for sarcopenia, our study revealed that malnutrition, low levels of physical activity, specific diseases (e.g., diabetes, osteoporosis), inflammation, polypharmacy (multiple medicines), BMI, and ageing are potential risk factor for sarcopenia in populations of the Nordic region. However, evidence on risk factors derived mainly from cross-sectional associations [27, 28, 30, 31, 34, 40, 44, 49,50,51], and only to a limited extend from longitudinal studies [24, 38, 43, 47]. Therefore, the associations between risk factors and sarcopenia should be interpreted with caution due to the possibility of reverse causality and confounding affecting the results. Moreover, our findings on risk factors mainly came from community-dwelling older adults, and only to a limited extend hospital and nursing home settings. We think that risk factors may vary depending on population characteristics (e.g., age, sex, health condition) and setting (e.g., hospital, nursing home, community). Therefore, we encourage researchers of the Nordic countries to perform well-designed prospective cohort studies in different settings to enhance the possibility to establish causal inference as well as understanding degree and direction of changes over time.
A recently published meta-analyses revealed a higher risk of having polypharmacy in Europe among individuals with sarcopenia compared to people without this condition [66]. A nationwide register-based study in Swedish population also showed that the prevalence of polypharmacy has increased in Sweden over the last decade [67]. Sarcopenia itself is associated with morbidity (identified by specific disease or inflammatory markers) and different health-related outcomes (e.g., disability) [7]; therefore, future research should investigate whether polypharmacy is a major factor to sarcopenia development [66]. Although we lack information on polypharmacy in Nordic countries other than Sweden, we encourage researchers in this region to examine the above research gap in their future studies.
According to previous studies physiological changes in ageing include systemic low-grade inflammation which results in insulin resistance, affect protein metabolism and leads to increased muscle wasting [68]. Acute and chronic disease may increase the inflammatory response and accelerate age-related loss of muscle mass and increase risk of sarcopenia [68, 69]. Hence, we think that special attention should be made by health care professionals particularly GPs to older adults with acute or chronic conditions to limit the risk of sarcopenia.
Literature from the Nordic countries also indicated that higher levels of physical activity and different dietary patterns (e.g., higher protein intake, fruit, vegetables, fibers) were associated with reduced risk of sarcopenia or improvement in indicators of sarcopenia. There was a large heterogeneity in the studied aspect which makes direct comparison of studies difficult. Nevertheless, according to findings from a recent systematic review of meta-analyses on sarcopenia the identified risk factors are in alignment with previously identified risk factors globally [70]. Other potential lifestyle-related risk factors suggested from the above meta-analysis included smoking and extreme sleep duration. However, we did not identify studies investigating these health behaviors in the Nordic populations. Therefore, high-quality cohort studies are needed to deeply understand such associations with the risk of sarcopenia.
In this current review, we only found three intervention studies in Nordic countries. However, two of them were sub-projects of big intervention programs, meaning that such studies were not designed explicitly for the prevention/treatment of sarcopenia. Therefore, explicit intervention studies on sarcopenia in this region is recommended.
We believe that on a global level, research on sarcopenia will carry on with nutrition, exercise, and understanding of molecular mechanisms. Furthermore, examining the link between sarcopenia and other medical conditions/diseases would be the next step [6]. In the Nordic countries, however, already performed studies have a basic and descriptive design, so that, well-designed research and advanced analyses are lacking. Hence, we recommend conducting large well-designed and adequately powered studies to (a) explore the scale of this age-related health issue on country and regional level, (b) investigate the patterns of physical activity and sedentary behavior to understand if this should be a target in older adults with SO and sarcopenia, (c) determine whether elderly populations are suffering from nutritional deficiency or are at risk of malnutrition. The latest can support further studies to assess the impact of combined physical activity and dietary intake, which are still lacking globally [6].
A previous systematic review on therapeutic strategies for SO revealed that exercise-based interventions (e.g., resistance training) reduced total adiposity and consequently improved body composition. However, evidence of other therapeutic strategies (e.g., nutritional supplementation) was limited due to scarcity of data and lack of unique definition for SO [69]. Therefore, authors suggested that more research should be done to clarify optimal treatment options for various age-groups and not only for older adults [14].
In our scoping review, the included studies, did not provide a status of either SO or the prevention/treatment methods in this region. We believe that SO is practically neglected in clinical practice and research as well, and this is mainly because it is difficult to separate it from general obesity. The consequence of lacking knowledge in this research area is that when older adults with SO are recommended weight loss- a frequently used strategy for management of general obesity- this may accelerate the loss of muscle mass and increase the severity of the sarcopenia [3]. Consequently, we think that this issue may have adverse effects both on patients (e.g., decreasing quality of their life) and on the health care system (e.g., increasing the health care demands) of this region. Therefore, we encourage researchers to perform cohort studies to understand the epidemiology and etiological basis of SO, which are poorly understood even on a global scale [8]. We think that the consensus definition on SO from the European Society for Clinical Nutrition and Metabolism (ESPEN) and European Association for the Study of Obesity (EASO) which was published in 2022 [3], can positively affect the ability to define studies on prevalence and prevention of SO. Besides, we recommend conducting further research to find the optimal treatment for SO and reduce its adverse consequences both at individual and society levels. Additionally, we think that the concepts of sarcopenia and SO might be somehow unfamiliar to health care personnel. Therefore, it is highly recommended that more information be provided to bring their attention to the significance of prevention, timely diagnosis, and treatment of these two aging disorders.
Strengths and limitations of the study
This is the first study providing an overview of available evidence on sarcopenia and SO among older adults in the Nordic countries. These countries have important similarities in welfare sectors and on a population level and we believe that our findings will be a significant benefit for researchers and health care providers to understand the knowledge gaps and plan for future studies in this geographical region. However, the current scoping review has limitations. This review was limited to studies among individuals more than 60 years old which may limit the overview of available research in this field, as well as understanding risk factors, confounders for prevention, and the potential for early detection of these two diseases in younger age population. The included cross-sectional studies in our review cannot provide information on causality of the associations.
Conclusion
Sarcopenia and SO are generally prevalent syndromes among older adults in Nordic countries, even though the prevalence of them varies according to the criteria for definition, population, and setting. Research among older adults with SO was very limited in this region. Besides, studies on risk factors were primarily cross-sectional and only few intervention studies were identified. Therefore, we encourage researchers performing well-designed studies (e.g., prospective cohorts) to understand the epidemiology and etiological basis of these two age-related disorders. For the next step, implementation of interventions targeting risk factors (e.g., combined physical activity and dietary intake) and evaluating of their impact on prevention or treatment of sarcopenia and SO is recommended. Furthermore, for the comprehensive advancement of muscle health in older adults, we recommend implementing interventions directed at health care personnel and encouraging more collaboration among clinicians, professional societies, researchers, and policy makers to ensure comprehensive and effective approach to health care initiatives.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- SO:
-
sarcopenic obesity
- WOS:
-
Web of science
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- EWGSOP2:
-
European Working Group on Sarcopenia in Older People in the updated version from 2019
- FNIH:
-
National Institutes of Health Sarcopenia Project definition
- DXA:
-
Dual energy X-ray absorptiometry
- BIA:
-
Bioelectrical Impedance Analysis
- BIS:
-
Bioimpedance Spectroscopy
- CT:
-
Computed Tomography
- CTA:
-
Computed Tomography Angiogram
- WHO:
-
World Health Organization
- GP:
-
General Practitioner
- RCT:
-
Randomized Controlled Trial
- COPD:
-
Chronic Obstructive Pulmonary Disease
- EASO:
-
European Association for the Study of Obesity
References
United, Nations, Department of Economic and Social Affairs., Population Division (2019). World Population Prospects 2019: Highlights (ST/ESA/SER.A/423).
United, Nations, Department of Economic and Social Affairs., Population Division (2019). World Population Ageing 2019: Highlights (ST/ESA/SER.A/430).
Donini LM, Busetto L, Bischoff SC, Cederholm T, Ballesteros-Pomar MD, Batsis JA, Bauer JM, Boirie Y, Cruz-Jentoft AJ, Dicker D, Frara S, Frühbeck G, Genton L, Gepner Y, Giustina A, Gonzalez MC, Han HS, Heymsfield SB, Higashiguchi T, Laviano A, Lenzi A, Nyulasi I, Parrinello E, Poggiogalle E, Prado CM, Salvador J, Rolland Y, Santini F, Serlie MJ, Shi H, Sieber CC, Siervo M, Vettor R, Villareal DT, Volkert D, Yu J, Zamboni M, Barazzoni R. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO Consensus Statement. Obes Facts. 2022;15(3):321–35. doi: 10.1159/000521241. Epub 2022 Feb 23. PMID: 35196654; PMCID: PMC9210010.
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M, Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. https://doi.org/10.1093/ageing/afy169. Erratum in: Age Ageing. 2019;48(4):601. PMID: 30312372; PMCID: PMC6322506.
Cylus J, Figueras J, Normand C. Will population ageing spell the end of the welfare state? A review of evidence and policy options [Internet]. Sagan A, Richardson E, North J, White C, editors. Copenhagen (Denmark): European Observatory on Health Systems and Policies; 2019. PMID: 31820887.
Yuan D, Jin H, Liu Q, Zhang J, Ma B, Xiao W, Li Y. Publication trends for Sarcopenia in the World: a 20-Year bibliometric analysis. Front Med (Lausanne). 2022;9:802651. https://doi.org/10.3389/fmed.2022.802651. PMID: 35223902; PMCID: PMC8873525.
Marengoni A, Angleman S, Melis R, Mangialasche F, Karp A, Garmen A, Meinow B, Fratiglioni L. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10(4):430–9. https://doi.org/10.1016/j.arr.2011.03.003. Epub 2011 Mar 23. PMID: 21402176.
Gao Q, Mei F, Shang Y, Hu K, Chen F, Zhao L, Ma B. Global prevalence of sarcopenic obesity in older adults: a systematic review and meta-analysis. Clin Nutr. 2021;40(7):4633–41. https://doi.org/10.1016/j.clnu.2021.06.009. Epub 2021 Jun 21. PMID: 34229269.
Molino S, Dossena M, Buonocore D, Verri M. Sarcopenic obesity: an appraisal of the current status of knowledge and management in elderly people. J Nutr Health Aging. 2016;20(7):780-8. https://doi.org/10.1007/s12603-015-0631-8. PMID: 27499312.
Khadra D, Itani L, Tannir H, Kreidieh D, El Masri D, El Ghoch M. Association between sarcopenic obesity and higher risk of type 2 diabetes in adults: a systematic review and meta-analysis. World J Diabetes. 2019;10(5):311–23. https://doi.org/10.4239/wjd.v10.i5.311. PMID: 31139318; PMCID: PMC6522758.
Aggio DA, Sartini C, Papacosta O, Lennon LT, Ash S, Whincup PH, Wannamethee SG, Jefferis BJ. Cross-sectional associations of objectively measured physical activity and sedentary time with Sarcopenia and sarcopenic obesity in older men. Prev Med. 2016;91:264–72. Epub 2016 Aug 26. PMID: 27575317; PMCID: PMC5061552.
Rossi AP, Rubele S, Calugi S, Caliari C, Pedelini F, Soave F, Chignola E, Vittoria Bazzani P, Mazzali G, Dalle Grave R, Zamboni M. Weight cycling as a risk factor for low muscle mass and strength in a population of males and females with obesity. Obesity (Silver Spring). 2019;27(7):1068–1075. https://doi.org/10.1002/oby.22493. PMID: 31231958.
Dent E, Morley JE, Cruz-Jentoft AJ, Arai H, Kritchevsky SB, Guralnik J, Bauer JM, Pahor M, Clark BC, Cesari M, Ruiz J, Sieber CC, Aubertin-Leheudre M, Waters DL, Visvanathan R, Landi F, Villareal DT, Fielding R, Won CW, Theou O, Martin FC, Dong B, Woo J, Flicker L, Ferrucci L, Merchant RA, Cao L, Cederholm T, Ribeiro SML, Rodríguez-Mañas L, Anker SD, Lundy J, Gutiérrez Robledo LM, Bautmans I, Aprahamian I, Schols JMGA, Izquierdo M, Vellas B. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22(10):1148–1161. https://doi.org/10.1007/s12603-018-1139-9. PMID: 30498820.
Poggiogalle E, Parrinello E, Barazzoni R, Busetto L, Donini LM. Therapeutic strategies for sarcopenic obesity: a systematic review. Curr Opin Clin Nutr Metab Care. 2021;24(1):33–41. https://doi.org/10.1097/MCO.0000000000000714. PMID: 33323715.
Petermann-Rocha F, Balntzi V, Gray SR, Lara J, Ho FK, Pell JP, Celis-Morales C. Global prevalence of Sarcopenia and severe Sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2022;13(1):86–99. https://doi.org/10.1002/jcsm.12783. Epub 2021 Nov 23. PMID: 34816624; PMCID: PMC8818604.
Prado CM, Wells JC, Smith SR, Stephan BC, Siervo M. Sarcopenic obesity: a critical appraisal of the current evidence. Clin Nutr. 2012;31(5):583–601. https://doi.org/10.1016/j.clnu.2012.06.010. Epub 2012 Jul 17. PMID: 22809635.
Balaj M, Huijts T, McNamara CL, Stornes P, Bambra C, Eikemo TA. Non-communicable diseases and the social determinants of health in the nordic countries: findings from the European Social Survey (2014) special module on the social determinants of health. Scand J Public Health. 2017;45(2):90–102. Epub 2017 Jan 27. PMID: 28128015.
Nordic Burden of Disease Collaborators. Life expectancy and disease burden in the Nordic countries: results from the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet Public Health. 2019;4(12): e658-e669. doi: 10.1016/S2468-2667(19)30224-5. Epub 2019 Nov 20. PMID: 31759894; PMCID: PMC7098475.
Stockmarr A, Hejgaard T, Matthiessen J. Obesity prevention in the Nordic Countries. Curr Obes Rep. 2016;5(2):156 – 65. https://doi.org/10.1007/s13679-016-0206-y. PMID: 27033877.
Cuadrado A, Stjernberg M, Huynh D. Active and healthy ageing: heterogenous perspectives and nordic indicators. Nordens välfärdscenter/Nordic Welfare Centre; 2022.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336 – 41. https://doi.org/10.1016/j.ijsu.2010.02.007. Epub 2010 Feb 18. Erratum in: Int J Surg. 2010;8(8):658. PMID: 20171303.
Sallfeldt ES, Mallmin H, Karlsson MK, Mellström D, Hailer NP, Ribom EL. Sarcopenia prevalence and incidence in older men - a MrOs Sweden study. Geriatr Nurs. 2023 Mar-Apr;50:102–8. https://doi.org/10.1016/j.gerinurse.2023.01.003. Epub 2023 Feb 10. PMID: 36774676.
Sääksjärvi K, Härkänen T, Stenholm S, Schaap L, Lundqvist A, Koskinen S, Borodulin K, Visser M. Probable Sarcopenia, obesity, and risk of all-cause mortality: a pooled analysis of 4,612 participants. Gerontology. 2023;69(6):706–15. Epub 2023 Jan 30. PMID: 36716714.
Karlsson M, Becker W, Cederholm TE, Byberg L. A posteriori dietary patterns in 71-year-old Swedish men and the prevalence of Sarcopenia 16 years later. Br J Nutr Camb Univ Press. 2022;128(5):909–20. https://doi.org/10.1017/S0007114521003901.
Dolin TG, Mikkelsen MK, Jakobsen HL, Vinther A, Zerahn B, Nielsen DL, Johansen JS, Lund CM, Suetta C. The prevalence of Sarcopenia and cachexia in older patients with localized colorectal cancer. J Geriatr Oncol. 2023;14(1):101402. Epub 2022 Nov 21. PMID: 36424269.
Paajanen P, Lindström I, Oksala N, Väärämäki S, Saari P, Mäkinen K, Kärkkäinen JM. Radiographically quantified Sarcopenia and traditional cardiovascular risk assessment in predicting long-term mortality after endovascular aortic repair. J Vasc Surg. 2022;76(4):908–e9152. Epub 2022 Mar 31. PMID: 35367563.
Sobestiansky S, Åberg AC, Cederholm T. Sarcopenia and malnutrition in relation to mortality in hospitalised patients in geriatric care - predictive validity of updated diagnoses. Clin Nutr ESPEN. 2021;45:442–8. Epub 2021 Jul 16. PMID: 34620352.
Papaioannou KG, Nilsson A, Nilsson LM, Kadi F. Healthy eating is Associated with Sarcopenia Risk in physically active older adults. Nutrients. 2021;13(8):2813. https://doi.org/10.3390/nu13082813. PMID: 34444973; PMCID: PMC8401667.
Wallengren O, Bosaeus I, Frändin K, Lissner L, Falk Erhag H, Wetterberg H, Rydberg Sterner T, Rydén L, Rothenberg E, Skoog I. Comparison of the 2010 and 2019 diagnostic criteria for Sarcopenia by the European Working Group on Sarcopenia in Older people (EWGSOP) in two cohorts of Swedish older adults. BMC Geriatr. 2021;21(1):600. https://doi.org/10.1186/s12877-021-02533-y. PMID: 34702174; PMCID: PMC8547086.
Scott D, Johansson J, Gandham A, Ebeling PR, Nordstrom P, Nordstrom A. Associations of accelerometer-determined physical activity and sedentary behavior with Sarcopenia and incident falls over 12 months in community-dwelling Swedish older adults. J Sport Health Sci. 2021;10(5):577–84. Epub 2020 Feb 5. PMID: 34088651; PMCID: PMC8500807.
Veen J, Montiel-Rojas D, Nilsson A, Kadi F. Engagement in muscle-strengthening activities lowers Sarcopenia Risk in older adults already adhering to the Aerobic Physical Activity guidelines. Int J Environ Res Public Health. 2021;18(3):989. https://doi.org/10.3390/ijerph18030989. PMID: 33499423; PMCID: PMC7908493.
Mølmen KS, Hammarström D, Pedersen K, Lian Lie AC, Steile RB, Nygaard H, Khan Y, Hamarsland H, Koll L, Hanestadhaugen M, Eriksen AL, Grindaker E, Whist JE, Buck D, Ahmad R, Strand TA, Rønnestad BR, Ellefsen S. Vitamin D3 supplementation does not enhance the effects of resistance training in older adults. J Cachexia Sarcopenia Muscle. 2021;12(3):599–628. https://doi.org/10.1002/jcsm.12688. Epub 2021 Mar 31. PMID: 33788419; PMCID: PMC8200443.
Simonsen C, Kristensen TS, Sundberg A, Wielsøe S, Christensen J, Hansen CP, Burgdorf SK, Suetta C, de Heer P, Svendsen LB, Achiam MP, Christensen JF. Assessment of Sarcopenia in patients with upper gastrointestinal tumors: prevalence and agreement between computed tomography and dual-energy x-ray absorptiometry. Clin Nutr. 2021;40(5):2809–16. Epub 2021 Mar 26. PMID: 33933747.
Faxén-Irving G, Luiking Y, Grönstedt H, Franzén E, Seiger Å, Vikström S, Wimo A, Boström AM, Cederholm T. Do malnutrition, sarcopenia and frailty overlap in nursing-home residents? J Frailty Aging. 2021;10(1):17–21. https://doi.org/10.14283/jfa.2020.45. PMID: 33331617.
Björkman MP, Suominen MH, Kautiainen H, Jyväkorpi SK, Finne-Soveri HU, Strandberg TE, Pitkälä KH, Tilvis RS. Effect of protein supplementation on physical performance in older people with sarcopenia-a randomized controlled trial. J Am Med Dir Assoc. 2020;21(2):226–e2321. Epub 2019 Nov 14. PMID: 31734121.
Jyväkorpi SK, Urtamo A, Kivimäki M, Strandberg TE. Macronutrient composition and sarcopenia in the oldest-old men: the Helsinki businessmen study (HBS). Clin Nutr. 2020;39(12):3839–41. https://doi.org/10.1016/j.clnu.2020.04.024. Epub 2020 Apr 24. PMID: 32376097.
Probert N, Lööw A, Akner G, Wretenberg P, Andersson ÅG. A comparison of patients with hip fracture, ten years apart: morbidity, malnutrition and sarcopenia. J Nutr Health Aging. 2020;24(8):870–877. https://doi.org/10.1007/s12603-020-1408-2. PMID: 33009538.
Sjöblom S, Sirola J, Rikkonen T, Erkkilä AT, Kröger H, Qazi SL, Isanejad M. Interaction of recommended levels of physical activity and protein intake is associated with greater physical function and lower fat mass in older women: Kuopio osteoporosis risk Factor- (OSTPRE) and fracture-Prevention Study. Br J Nutr. 2020;123(7):826–39. Epub 2020 Jan 8. PMID: 31910914; PMCID: PMC7054249.
von Berens Å, Obling SR, Nydahl M, Koochek A, Lissner L, Skoog I, Frändin K, Skoglund E, Rothenberg E, Cederholm T. Sarcopenic obesity and associations with mortality in older women and men - a prospective observational study. BMC Geriatr. 2020;20(1):199. https://doi.org/10.1186/s12877-020-01578-9. PMID: 32517653; PMCID: PMC7285448.
Nielsen BR, Andersen HE, Haddock B, Hovind P, Schwarz P, Suetta C. Prevalence of muscle dysfunction concomitant with osteoporosis in a home-dwelling Danish population aged 65–93 years -the Copenhagen Sarcopenia Study. Exp Gerontol. 2020;138:110974. https://doi.org/10.1016/j.exger.2020.110974. Epub 2020 May 25. PMID: 32464171.
Van Ancum JM, Alcazar J, Meskers CGM, Nielsen BR, Suetta C, Maier AB. Impact of using the updated EWGSOP2 definition in diagnosing Sarcopenia: a clinical perspective. Arch Gerontol Geriatr 2020 Sep-Oct;90:104125. https://doi.org/10.1016/j.archger.2020.104125. Epub 2020 May 23. PMID: 32534364.
Björkman M, Jyväkorpi SK, Strandberg TE, Pitkälä KH, Tilvis RS. Sarcopenia indicators as predictors of functional decline and need for care among older people. J Nutr Health Aging. 2019;23(10):916–922. https://doi.org/10.1007/s12603-019-1280-0. PMID: 31781719.
Björkman MP, Pitkala KH, Jyväkorpi S, Strandberg TE, Tilvis RS. Bioimpedance analysis and physical functioning as mortality indicators among older sarcopenic people. Exp Gerontol. 2019;122:42–6. https://doi.org/10.1016/j.exger.2019.04.012. Epub 2019 Apr 24. PMID: 31026498.
Olesen SS, Büyükuslu A, Køhler M, Rasmussen HH, Drewes AM. Sarcopenia associates with increased hospitalization rates and reduced survival in patients with chronic pancreatitis. Pancreatology. 2019;19(2):245–51. https://doi.org/10.1016/j.pan.2019.01.006. Epub 2019 Jan 14. PMID: 30665702.
Sobestiansky S, Michaelsson K, Cederholm T. Sarcopenia prevalence and associations with mortality and hospitalisation by various sarcopenia definitions in 85–89 year old community-dwelling men: a report from the ULSAM study. BMC Geriatr. 2019;19(1):318. https://doi.org/10.1186/s12877-019-1338-1. PMID: 31747923; PMCID: PMC6864927.
Vikberg S, Sörlén N, Brandén L, Johansson J, Nordström A, Hult A, Nordström P. Effects of resistance training on functional strength and muscle mass in 70-Year-old individuals with pre-sarcopenia: a randomized controlled trial. J Am Med Dir Assoc. 2019;20(1):28–34. Epub 2018 Nov 7. PMID: 30414822.
Isanejad M, Sirola J, Mursu J, Rikkonen T, Kröger H, Tuppurainen M, Erkkilä AT. Association of the baltic sea and mediterranean diets with indices of sarcopenia in elderly women, OSPTRE-FPS study. Eur J Nutr. 2018;57(4):1435–48. https://doi.org/10.1007/s00394-017-1422-2. Epub 2017 Mar 16. PMID: 28303397.
Mikkola TM, von Bonsdorff MB, Salonen MK, Simonen M, Pohjolainen P, Osmond C, Perälä MM, Rantanen T, Kajantie E, Eriksson JG. Body composition as a predictor of physical performance in older age: a ten-year follow-up of the Helsinki Birth Cohort Study. Arch Gerontol Geriatr. 2018 Jul-Aug;77:163–8. doi: 10.1016/j.archger.2018.05.009. Epub 2018 May 14. PMID: 29783137; PMCID: PMC5994345.
Ottestad I, Ulven SM, Øyri LKL, Sandvei KS, Gjevestad GO, Bye A, Sheikh NA, Biong AS, Andersen LF, Holven KB. Reduced plasma concentration of branched-chain amino acids in sarcopenic older subjects: a cross-sectional study. Br J Nutr. 2018;120(4):445–53. Epub 2018 Jun 18. PMID: 29909813.
Steihaug OM, Gjesdal CG, Bogen B, Kristoffersen MH, Lien G, Ranhoff AH. Sarcopenia in patients with hip fracture: a multicenter cross-sectional study. PLoS ONE. 2017;12(9):e0184780. https://doi.org/10.1371/journal.pone.0184780. PMID: 28902873; PMCID: PMC5597226.
Jacobsen EL, Brovold T, Bergland A, Bye A. Prevalence of factors associated with malnutrition among acute geriatric patients in Norway: a cross-sectional study. BMJ Open. 2016;6(9):e011512. https://doi.org/10.1136/bmjopen-2016-011512. PMID: 27601491; PMCID: PMC5020767.
Jansen RB, Christensen TM, Bülow J, Rørdam L, Holstein PE, Svendsen OL. Sarcopenia and body composition in diabetic Charcot osteoarthropathy. J Diabetes Complications. 2015 Sep-Oct;29(7):937–42. https://doi.org/10.1016/j.jdiacomp.2015.05.020. Epub 2015 Jun 5. PMID: 26139557.
Frost M, Nielsen TL, Brixen K, Andersen M. Peak muscle mass in young men and Sarcopenia in the ageing male. Osteoporos Int. 2015;26(2):749–56. https://doi.org/10.1007/s00198-014-2960-6. Epub 2014 Nov 22. PMID: 25416073.
Patil R, Uusi-Rasi K, Pasanen M, Kannus P, Karinkanta S, Sievänen H. Sarcopenia and osteopenia among 70-80-year-old home-dwelling finnish women: prevalence and association with functional performance. Osteoporos Int. 2013;24(3):787–96. https://doi.org/10.1007/s00198-012-2046-2. Epub 2012 Jun 12. PMID: 22688541.
Papadopoulou SK, Tsintavis P, Potsaki P, Papandreou D. Differences in the prevalence of sarcopenia in community-dwelling, nursing home and hospitalized individuals. a systematic review and meta-analysis. J Nutr Health Aging. 2020;24(1):83–90. https://doi.org/10.1007/s12603-019-1267-x. PMID: 31886813.
Mayhew AJ, Amog K, Phillips S, Parise G, McNicholas PD, de Souza RJ, Thabane L, Raina P. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: a systematic review and meta-analyses. Age Ageing. 2019;48(1):48–56. https://doi.org/10.1093/ageing/afy106. PMID: 30052707.
Cao M, Lian J, Lin X, Liu J, Chen C, Xu S, Ma S, Wang F, Zhang N, Qi X, Xu G, Peng N. Prevalence of Sarcopenia under different diagnostic criteria and the changes in muscle mass, muscle strength, and physical function with age in Chinese old adults. BMC Geriatr. 2022;22(1):889. https://doi.org/10.1186/s12877-022-03601-7. PMID: 36418979; PMCID: PMC9682713.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M, European working group on sarcopenia in older people. sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39(4):412–23. https://doi.org/10.1093/ageing/afq034. Epub 2010 Apr 13. PMID: 20392703; PMCID: PMC2886201.
Verstraeten LMG, de Haan NJ, Verbeet E, van Wijngaarden JP, Meskers CGM, Maier AB. Handgrip strength rather than chair stand test should be used to diagnose s in geriatric rehabilitation inpatients: restoring health of acutely unwell adulTs (RESORT). Age Ageing. 2022;51(11):afac242. https://doi.org/10.1093/ageing/afac242. PMID: 36413590; PMCID: PMC9681126.
Cheng KY, Chow SK, Hung VW, Wong CH, Wong RM, Tsang CS, Kwok T, Cheung WH. Diagnosis of sarcopenia by evaluating skeletal muscle mass by adjusted bioimpedance analysis validated with dual-energy X-ray absorptiometry. J Cachexia Sarcopenia Muscle. 2021;12(6):2163–73. Epub 2021 Oct 4. PMID: 34609065; PMCID: PMC8718029.
Sousa-Santos AR, Barros D, Montanha TL, Carvalho J, Amaral TF. Which is the best alternative to estimate muscle mass for sarcopenia diagnosis when DXA is unavailable? Arch Gerontol Geriatr. 2021 Nov-Dec;97:104517. https://doi.org/10.1016/j.archger.2021.104517. Epub 2021 Sep 3. PMID: 34547538.
González Correa CH, Marulanda Mejía F, Castaño González PA, Vidarte Claros JA, Castiblanco Arroyabe HD. Bioelectrical impedance analysis and dual x-ray absorptiometry agreement for skeletal muscle mass index evaluation in sarcopenia diagnosis. Physiol Meas. 2020;41(6):064005. https://doi.org/10.1088/1361-6579/ab8e5f. PMID: 32348971.
Hwang B, Lim JY, Lee J, Choi NK, Ahn YO, Park BJ. Prevalence rate and associated factors of sarcopenic obesity in Korean elderly population. J Korean Med Sci. 2012;27(7):748–55. https://doi.org/10.3346/jkms.2012.27.7.748. Epub 2012 Jun 29. PMID: 22787369; PMCID: PMC3390722.
Kera T, Kawai H, Hirano H, Kojima M, Fujiwara Y, Ihara K, Obuchi S. Differences in body composition and physical function related to pure Sarcopenia and sarcopenic obesity: a study of community-dwelling older adults in Japan. Geriatr Gerontol Int. 2017;17(12):2602–9. https://doi.org/10.1111/ggi.13119. Epub 2017 Jun 28. PMID: 28657168.
Aibar-Almazán A, Martínez-Amat A, Cruz-Díaz D, Jiménez-García JD, Achalandabaso A, Sánchez-Montesinos I, de la Torre-Cruz M, Hita-Contreras F. Sarcopenia and sarcopenic obesity in Spanish community-dwelling middle-aged and older women: Association with balance confidence, fear of falling and fall risk. Maturitas. 2018;107:26–32. Epub 2017 Oct 7. PMID: 29169576.
Prokopidis K, Giannos P, Reginster JY, Bruyere O, Petrovic M, Cherubini A, Triantafyllidis KK, Kechagias KS, Dionyssiotis Y, Cesari M, Ibrahim K, Scott D, Barbagallo M, Veronese N, the Task Force on Pharmaceutical Strategy of the European Geriatric Medicine Society (EuGMS). Special interest group in Systematic Reviews and Meta-analyses and sarcopenia is associated with a greater risk of polypharmacy and number of medications: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2023;14(2):671–683. https://doi.org/10.1002/jcsm.13190. Epub 2023 Feb 13. PMID: 36781175; PMCID: PMC10067503.
Zhang N, Sundquist J, Sundquist K, Ji J. An increasing Trend in the prevalence of polypharmacy in Sweden: a nationwide register-based study. Front Pharmacol. 2020;11:326. https://doi.org/10.3389/fphar.2020.00326. PMID: 32265705; PMCID: PMC7103636.
Dalle S, Rossmeislova L, Koppo K. The role of inflammation in age-related sarcopenia. Front Physiol. 2017;8:1045. https://doi.org/10.3389/fphys.2017.01045. PMID: 29311975; PMCID: PMC5733049.
Riuzzi F, Sorci G, Arcuri C, Giambanco I, Bellezza I, Minelli A, Donato R. Cellular and molecular mechanisms of sarcopenia: the S100B perspective. J Cachexia Sarcopenia Muscle. 2018;9(7):1255–68. https://doi.org/10.1002/jcsm.12363. Epub 2018 Nov 30. PMID: 30499235; PMCID: PMC6351675.
Yuan S, Larsson SC. Epidemiology of sarcopenia: prevalence, risk factors, and consequences. Metabolism. 2023;144:155533. https://doi.org/10.1016/j.metabol.2023.155533. Epub 2023 Mar 11. PMID: 36907247.
Acknowledgements
Not applicable.
Funding
Open access funding provided by University of Southern Denmark
This work was done without any fund.
Author information
Authors and Affiliations
Contributions
FB conceived and designed the review, participated in literature review, data extraction, interpretation of the results and wrote the manuscript. SFB designed the review, participated in literature review, data extraction, and revised the manuscript. TT, JBN and JS contributed to the conception of the study and revised the manuscript critically. All the authors approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Baygi, F., Buhl, S.F., Thilsing, T. et al. Sarcopenia and sarcopenic obesity among older adults in the nordic countries: a scoping review. BMC Geriatr 24, 421 (2024). https://doi.org/10.1186/s12877-024-04970-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-024-04970-x