- Software
- Open access
- Published:
AGGRESCAN: a server for the prediction and evaluation of "hot spots" of aggregation in polypeptides
BMC Bioinformatics volume 8, Article number: 65 (2007)
Abstract
Background
Protein aggregation correlates with the development of several debilitating human disorders of growing incidence, such as Alzheimer's and Parkinson's diseases. On the biotechnological side, protein production is often hampered by the accumulation of recombinant proteins into aggregates. Thus, the development of methods to anticipate the aggregation properties of polypeptides is receiving increasing attention. AGGRESCAN is a web-based software for the prediction of aggregation-prone segments in protein sequences, the analysis of the effect of mutations on protein aggregation propensities and the comparison of the aggregation properties of different proteins or protein sets.
Results
AGGRESCAN is based on an aggregation-propensity scale for natural amino acids derived from in vivo experiments and on the assumption that short and specific sequence stretches modulate protein aggregation. The algorithm is shown to identify a series of protein fragments involved in the aggregation of disease-related proteins and to predict the effect of genetic mutations on their deposition propensities. It also provides new insights into the differential aggregation properties displayed by globular proteins, natively unfolded polypeptides, amyloidogenic proteins and proteins found in bacterial inclusion bodies.
Conclusion
By identifying aggregation-prone segments in proteins, AGGRESCAN http://bioinf.uab.es/aggrescan/ shall facilitate (i) the identification of possible therapeutic targets for anti-depositional strategies in conformational diseases and (ii) the anticipation of aggregation phenomena during storage or recombinant production of bioactive polypeptides or polypeptide sets.
Background
Protein aggregation has become a key topic in both biotechnological and medical sciences [1, 2]. It constitutes the main bottleneck in protein production, narrowing the spectrum of relevant polypeptides obtained by recombinant techniques [3]; it reduces the shelf life and increases the immunogenicity of polypeptidic drugs [4]; and it is associated with an increasing number of critical human diseases including Alzheimer's disease, spongiform encephalopaties, type II diabetes mellitus and Parkinson's disease [5–8].
In the last decade data have begun to accumulate suggesting that the composition and the primary structure of a polypeptide determine to a large extent its propensity to aggregate and that small changes may have a huge impact on solubility. The ability to predict the aggregation propensity of a protein from its sequence would be of much value, for example, in the control of unwanted protein deposition events through specific sequence targeted therapeutics or in the discovery of more soluble variants of proteins of biotechnological interest. It is commonly assumed that not all regions of a polypeptide are equally important in determining its aggregation tendency. In this context, some authors have recently proved that very short specific amino acid stretches can act as facilitators or inhibitors of amyloid fibril formation [9, 10]. These relevant regions are usually known as aggregation "hot spots" (HS) and their presence has been described in most of the peptides and proteins underlying neurodegenerative and systemic amyloidogenic disorders [11].
In previous work we exploited the experimental data obtained from a system in vivo that uses the β-amyloid peptide as model to derive a simple approach for the detection of "hot spots" of aggregation [12, 13]. This approach permitted the identification of aggregation-prone segments in several unstructured and globular disease-linked polypeptides and the prediction of the effect of disease-linked mutations in some of these polypeptides. Here, we describe a software and web interface (AGGRESCAN) that implement this approach and extend it to the general prediction of aggregation "hot spots" and the evaluation of their contribution to the differential aggregation behaviour of polypeptides. In addition to enabling the simultaneous analysis of a large number of sequences, AGGRESCAN introduces a new set of functions and descriptors for the identification of "hot spots" of aggregation and the determination of their relevance within the parent sequence.
Implementation
Approach
Recent findings in the study of protein aggregation indicate that not all the polypeptides share the same aggregation propensities and that there exists specific continuous protein segments that can nucleate the aggregation process when exposed to solvent [9, 10], suggesting a sequence-dependence of aggregation propensities. At the same time, it has been shown that the same physicochemical principles underlie the aggregation propensities of different polypeptides from unfolded states [14]. According to these assumptions one may expect that the conclusions obtained from the study of a relevant nucleating sequence, or "hot spot" of aggregation, in its natural polypeptidic context could apply to other unrelated proteins. Using an in vivo reporter method to study a "hot spot" in the central hydrophobic core of Aβ we calculated the effect of single point mutations on the aggregation propensities of the peptide within the cell. The results were used to approximate the in vivo intrinsic aggregation propensities of natural amino acids when located in an aggregation-prone sequence stretch [12] (see additional file 1). This information was subsequently used to generate an aggregation profile for any protein sequence under study to detect those regions with high aggregation propensities. Comparison of the theoretically calculated changes in aggregation propensities between a wild type sequence and different mutants serves also as a tool to predict the behavior of the mutant forms. Albeit the basic simplicity of this phenomenological model, it predicts, at least qualitatively, both the presence of experimentally validated "hot spots" and the variations in aggregation propensity introduced by mutations in some disease-related polypeptides [13].
System description
AGGRESCAN is a web-based tool with a computing core coded in C and a front end written in a combination of html and perl cgi. Development of AGGRESCAN was carried out under Mandriva Linux LE2005 and the service is currently running under Mandrake Linux 9.0 on a Pentium 4 1300 MHz (willamette) with 1GB RDRAM.
For each polypeptide sequence input, AGGRESCAN calculates and reports: i) an aggregation-propensity value for each residue in the sequence and a graphical representation of the profile for the entire polypeptide; ii) the areas of profile peaks over a precalculated threshold and a graphical representation of peak-area values; iii) putative aggregation "hot spots", identified from the polypeptide's aggregation profile.
Input
The polypeptide sequence(s) can be typed or pasted on screen using FASTA format. Despite supporting up to 100 characters for name entries, use of very long names is discouraged as it disturbs the visualization of the output. Sequence entries may not contain more than 2,000 residues and the letters must correspond to those associated to the 20 natural amino acids. If these two conditions are not satisfied an error message will appear on screen. White-space, enter and tab characters are ignored. Characters may be entered as lower and/or upper case, and so will remain in the output.
Processing
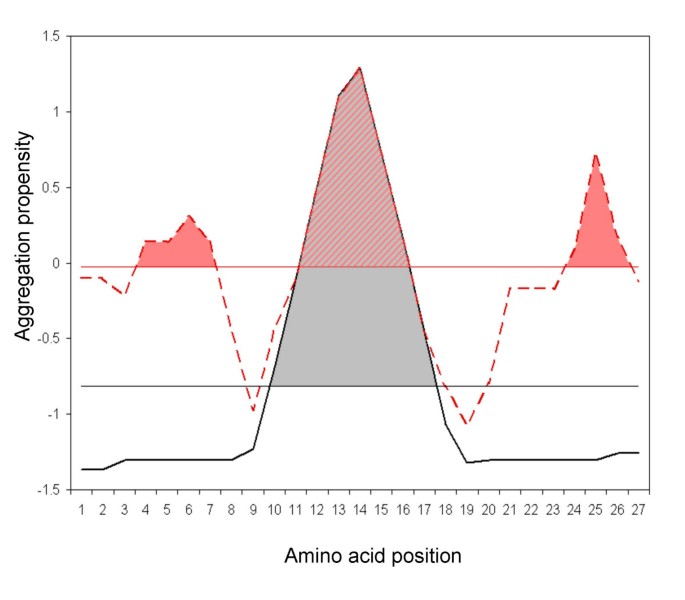
The calculations are based on aggregation-propensity values per amino acid (aaAV, or a3v) derived previously from experimental data [12]. The program calculates the a3v average (a4v) over a sliding window of a given length and assigns it to the central residue in the window. The size of the sliding window ([5, 7, 9], and [11] residues) was trained against a database of 57 amyloidogenic proteins, in which the location of "hot spots" was experimentally known. In general, the predictions of the overall aggregation-prone regions do not depend on the length of the used windows and only slightly affect their limits. There are, however, two remarkable exceptions: 1) The use of long windows on top of very short sequences results on excessive smoothing of the profile and experimentally different "hot spots" become grouped and masked and cannot be individualized in the prediction. 2) The use of short windows on top of very long sequences results in the appearance of a number of short experimentally non-relevant predicted "hot spots" with associated low areas. Thus, the procedure incorporates a ponderation of the window length relative to the size of the analyzed protein. The best predictions were obtained using a window size of 5 for < = 75 residues, 7 for < = 175, 9 for < = 300 and 11 for > 300, respectively, probably reflecting that for longer sequences larger "hot spots" are necessary in order to significantly increase their aggregation propensities, while short-stretches suffice for smaller peptides. To account for charge effects at the polypeptide's termini (NH3 + and COO-) a virtual residue is added to each side of the chain (residue 0 at the N-terminus and residue n+1 at the C-terminus, n being the original sequence length). The a3v of residue 0 equals the average a3v of the basic residues (K, R), while that of residue n+1 equals the average a3v of the acidic residues (D, E). The first window, ranging from residue 0 to residue 4, 6, 8 or 10 (depending on window size), will serve to assign an a4v to residue 2, 3, 4 or 5, respectively. Thus, the off-centre residues 1, 1–2, 1–3 or 1–4 may not have an associated a4v. This is solved by giving these residues the value corresponding to the first window centre. The same procedure is followed at the C-terminus. The "hot spot" threshold (HST) has been defined as the average of the a3v of the 20 amino acids weighted by their frequencies in the SwissProt database [15]. The aggregation profile (AP) of the polypeptide is defined by the complete sequence of a4v. The sum of a4v and the average of a3v over the entire sequence (a4vSS and a3vSA, respectively) are also calculated. A region in the polypeptide sequence is considered an aggregation "hot spot" (HS) if there are 5 or more sequentially continuous residues with an a4v larger than the HST and none of them is a proline (aggregation breaker) [16]. The average a4v in each "hot spot" is then calculated (a4vAHS). Finally, the area of the AP above the HST (AAT), the total area (TA, HST being the zero axis), and the area above the HST of each profile peak identified as "hot spot" (HSA) are integrated numerically using the trapezoidal rule (see additional file 2).
Output
With current service resources, the delay time between pressing the submit button and receiving the output on screen is of 10 minutes for an input set of 100 sequences of sizes between 40 and 1,000 residues. The output is structured in tables, one per sequence and an additional one with averages over all sequences, an excel-readable document with output values and a list of sequences sorted by normalized a4vSS for 100 residues (Na4vSS). The first row in the output contains the sequence names. The second row displays links to the three graphics produced per sequence, i.e., Profile graphic: AP (red), a3vSA (green), HST (blue); Area graphic: HSA (same value assigned to all residues in the "hot spot"); Normalized-Area graphic: normalized HSA for a 100-residue "hot spot" (NHSA). In the following rows we find the a3vSA, the number of "hot spots" identified (nHS), the normalized number of "hot spots" for 100 residues (NnHS), the AAT, the THSA, the TA, the AAT and THSA divided by the number of residues (AATr and THSAr, respectively), and Na4vSS. Finally, a row per residue is given with columns for the residue number, its one-letter code, a4v, HSA, NHSA, and a4vAHS (see additional file 3).
Results and Discussion
AGGRESCAN capabilities: Validation and Examples
Generation of protein aggregation profiles and prediction of aggregation "hot spots"
The prediction method implemented in AGGRESCAN has already allowed the identification of experimentally proved "hot spots" (HSs) in a set of both natively unfolded and globular pathogenic proteins: Aβ42 peptide, synuclein, amylin, prion protein, transthyretin, β2-microglobulin and lysozyme [12]. The main aims in the design of AGGRESCAN were the automation of this analysis for the study of large sets of polypeptide sequences, the introduction of new variables in the postprocessing of the aggregation profiles to provide a set of values that could be easily correlated with aggregation propensities and the presentation of results in a convenient and informative way. To further prove the general predictive ability of the method, the above-mentioned proteins, together with a new set of well studied protein sequences related to depositional diseases (aDan, aBri, apolipoproteins AI, AII, AIV, and CII, prolactin, insulin, Tau, fibrinogen, amyloid A, pulmonary surfactant protein, tropoelastin and medin), or shown to form amyloid in vitro (myoglobin, glycophorin A and amphoterin) have been analyzed with AGGRESCAN (Table 1). The predicted aggregation-prone protein regions have been validated by comparison to available experimental data on (i) regions known to promote aggregation, (ii) fragments known to aggregate in vivo (often after proteolysis) and (iii) synthetic short peptides shown to aggregate in vitro (references in Table 1). In the AGGRESCAN output, the sequence stretches with highest predicted aggregation propensity are shown in red in the peptide sequence column and appear as peaks in the Profile plots. The HS can be ranked according to their peak area (HSA) or normalized peak area (NHSA). Interestingly, protein segments that are experimentally known to be involved in aggregation are also found among the top ranked HS in their respective sequences based of the approach described here (Table 1), indicating that AGGRESCAN catches the main features underlying deposition in many conformational diseases. These results, together with previous experimental [10, 17–20] and theoretical [21–24] data, suggest that specific short polypeptide stretches effectively promote and/or modulate protein amyloid formation.
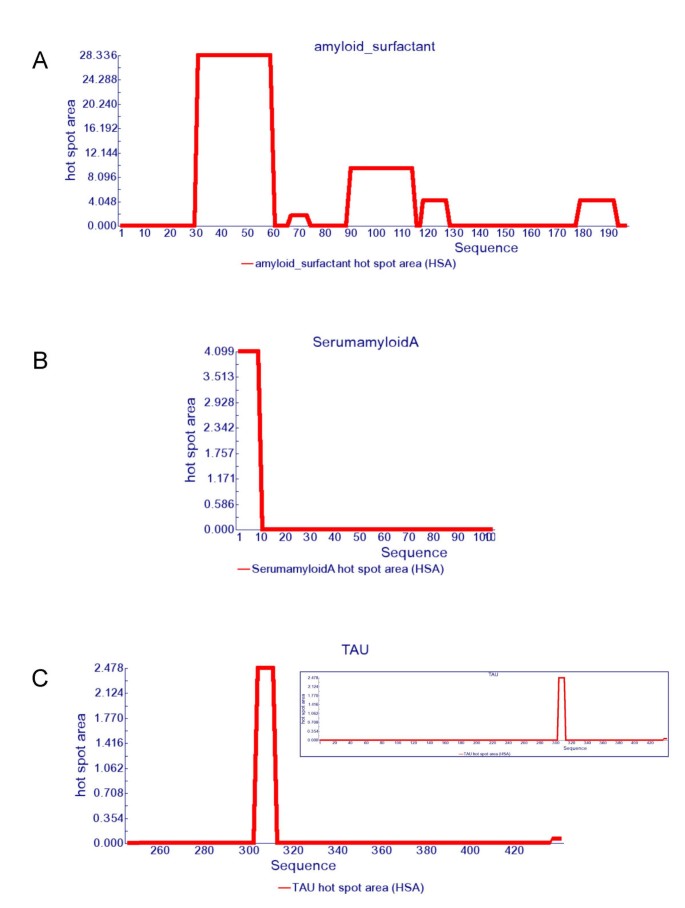
One remarkable example in the test set is lung surfactant protein C (SP-C). This protein is expressed as a 197-amino acid proprotein that is processed to the 35-amino acid mature peptide. This fragment is associated with the development of pulmonary alveolar proteinosis (PAP). The bronchoalveolar fluid from PAP patients is rich in insoluble SP-C aggregates which exhibit the characteristic properties of amyloids by Congo red staining and electron microscopy. Moreover, the isolated peptide has been shown to form amyloid fibrils in vitro [25]. In good agreement with this data, AGGRESCAN predicts the SP-C region within the precursor as the HS with the highest aggregation propensity (Figure 1).
Other two interesting molecules are serum amyloid A (SAA) and Tau proteins, involved in systemic amyloidosis and Alzheimer's disease, respectively. AGGRESCAN detects only one HS in SAA and a very dominant one in Tau (Figure 1). In both cases, these sequence stretches correspond to the unique regions in SAA and Tau proved to be relevant for amyloidosis [26, 27]. Importantly, the SAA and Tau sequences display highly negative Na4vSS values, -28.2 and -32.5 respectively. Although this suggests an overall low aggregation propensity, the presence of specific HS that can act as nucleation points from which the ordered fibrillar structure can be expanded under certain circumstances, turn these proteins amyloidogenic. Actually, Tau is an usually highly soluble microtubule-associated protein [28] but in Alzheimer's disease it aggregates into fibres with a tendency to form neurofibrillary tangles.
To date, only few 3D structures of amyloid assemblies at atomic resolution are available [29]. A crucial question is whether the formation of the tightly packed β-sheets observed in these structures is a generic backbone property or is dictated by the sequence. Interestingly enough, AGGRESCAN detects the presence of "hot spots" in most of the strands forming the intimate structure of the different protein fibrils (Table 2), providing additional support for the relevance of the primary structure on amyloid formation.
There are several computational approaches for detecting aggregation-prone regions and predicting polypeptide propensities for amyloid fibril formation. Some of them, including AGGRESCAN, rely on experimental or theoretical calculations of individual amino acid aggregation propensities and on the use of these values to scan protein sequences. The main difference between these algorithms is the way aggregation propensities are obtained. Pawar and co-workers proposed an aggregation scale based on phenomenological expressions relating protein intrinsic factors with the aggregation rates of a set point mutants scattered along acylphosphatase sequence and of a few other polypeptides [30]. As the fitting was done considering effects in both aggregation relevant and non-relevant regions, it is possible that the data do not necessarily reflect propensities within nucleating sequences. To address this point, Rojas Quijano and co-workers derived propensities from the analysis of the Tau-related amyloidogenic peptide Ac-VQIVYK-amide and its single site mutants Ac-VQIVXK-amide (X≠Cys) [19]. In AGGRESCAN, we somehow combine both approaches, in the sense that (i) propensities are calculated from the analysis of single mutants in a nucleating sequence (the central hydrophobic cluster of Aβ) which is perhaps the best well characterized aggregation-prone sequence in the literature and one of the few for which a high-resolution structure in the amyloid conformation is available, and (ii) we consider it in the context of the full length polypeptide (in fact fused to GFP, which acts as aggregation reporter) and not in an isolated manner as a short peptide. In addition, to the best of our knowledge our method is the only one in which aggregation propensities have been derived from experiments inside the cell, where the presence of the folding machinery might modulate the aggregation tendencies of polypeptides. Besides these three experimentally calculated propensity scales, Galzitskaya and co-workers have used the mean packing density for natural amino acid residues in protein structures, as a scale to predict amyloidogenic regions in proteins [31]. A comparative analysis of the four different scales shows that, despite these differences, there is a striking correlation between our in vivo obtained amino acid aggregation propensities and the others (Table 3), probably because they reflect a combination of properties characteristic of protein aggregation, such as hydrophobicity, secondary structure propensity or packing density. Importantly, although our method was not aimed at the specific identification of short amyloidogenic peptides, but rather of aggregation-prone sequences within natural proteins, AGGRESCAN identifies the presence of at least one hot spot in more than 80% of the amyloid forming sequences in a set of experimentally characterized peptide fragments of amyloidogenic proteins [32]. Also, using a database of six-residue peptides containing both amyloid formers and non-formers [32, 33] the receiver operator characteristic (ROC) curve for our method compares well with those obtained using structure-based data, such us packing density on protein structures or the 3D profile method, based on the threading of six-residue peptides through the known crystal structure of the cross-β spine formed by the peptide NNQQNY from Sup35 yeast prion [32] (Figure 2).
Comparative prediction performance of AGGRESCAN and structure-based methods. Comparative predictions of AGGRESCAN (solid circles), packing density profile [31] (no symbols), 3D Profile [32] using the NNQQNY template (solid squares) and 3D Profile using an ensemble of templates (empty squares). Predictions were tested in a Database of Fibril Formers and Non-Formers hexa-peptides. Predictions are shown as receiver-operator characteristic curves.
Overall, the success of different computational approaches in predicting aggregation-prone regions allows to propose that aggregation propensity in polypeptide chains is ultimately dictated by the sequence. As it happens with the native conformation of proteins, the sequence contains intrinsic information that is relevant for the regular structural arrangement within β-aggregates, implying that the mechanism of amyloid fibril formation is similar for different peptides and proteins.
Prediction of the effects of protein mutation on the aggregation propensity
Aggregation propensity varies sensibly with the composition and especially the sequence of the polypeptide, in such a way that single amino acid substitutions in proteins associated to depositional diseases result in many cases in changes in the global protein aggregation propensity and sometimes lead to premature or acute pathological symptoms. Predicting the effect of a mutation on the aggregation tendency of a protein could help to anticipate the implications of that mutation in disease development or assist the design, production and storage of more soluble variants of biotechnologically relevant proteins and peptides [34].
Several AGGRESCAN output variables can be used to predict the effect of sequence variations on the aggregation propensities of a given polypeptide. The change in the normalized a4v sum (Na4vSS) and Total Area (TA) are obvious indicators of changes in aggregation properties of the complete sequence due to point mutations. Nevertheless, a mutation will not always affect significantly the global profile and changes in the number of HS (nHS), in the area over the HS threshold (AAT) or in the area assigned to the HS regions (THSA), are also informative. The normalized values (relative to the number of residues) AATr, THSAr and NHSA should be used if mutations resulting in sequence deletions or insertions are considered. To asses the capability of AGGRESCAN to predict sequence-variation effects we compared the experimentally observed aggregation changes reported in the literature for a group of more than 50 protein mutations with the change in different AGGRESCAN output variables. The analysis indicates that Na4vSS is a good predictor for the effect on aggregation propensity changes in the polypeptide sequence on aggregation propensity (Table 4). The user has to take into account that a given mutation in a short protein is expected to have higher impact on aggregation that the same change in a longer sequence, where its effect can be more easily modulate by the rest of the sequence. These considerations are already included in the calculation of the Na4vSS values.
The algorithm predicts accurately a large set of natural and designed mutations of Aβ42 (the central hydrophobic region of this peptide was used for the derivation of the current a3v parameter set of AGGRESCAN) (Table 4). As an example, Figure 3 shows how the F19T mutation, which strongly decreases the deposition of Aβ42 [35], results in the loss of the central HS in this peptide. Interestingly, it also anticipates the lower aggregation propensity of Aβ40 and the recent observation that longer Aβisoforms possess increased aggregation propensities [36]. Several natural occurring mutations have also been shown to affect the aggregation rate of Tau [37–40]. The predicted changes in the respective Na4vSS correlate well with the experimental changes observed in these Tau variants (Table 4). Figure 3 shows the Area plot of wild type Tau and two of its mutants with highest, experimentally tested, aggregation propensities. The P301L substitution increases by 1,4 fold the area associated to the main "hot spot" in Tau. In addition, AGGRESCAN predicts the presence of a new HS in the S320F mutant, absent in the wild type form. This mutant is linked to tauthopaty, in which Tau accumulates in inclusion bodies [40].
Other disease-related protein mutants studied here are the recently described G4R and R68Stop of human Stefin B protein. These mutants have been related with the development of Myoclonus epilepsy of type 1. It has been described that R68Stop is more prone to aggregate than wild type Stefin, while the G4R mutant shows an opposite behavior, with a slower fibril formation rate [41]. In agreement with these experimental observations the algorithm predicts an increase in the Na4vSS associated to the R68Stop mutation and a decrease for the Gly4Arg change (Table 4).
Type 1 serum amyloid A protein (SAA1) is associated with Familial Mediterranean fever (FMF). FMF patients' genotypes are thought to correlate with the different phenotypes of the disease. A recent study [42] concludes that the gamma SAA1 allele is more frequently observed in the population devoid of amyloidosis, thus suggesting a protective effect of this allele on the development of the illness. In agreement with these results the AGGRESCAN analysis of amyloid A sequence variants predicts that the gamma variant misses a HS and has a lower Na4vSS than other alleles.
The Src homology 3 (SH3) domain of the p58 subunit of phosphatidyl-inositol-3 -kinase (PI3-SH3) is one of the best-characterized examples of a small globular protein unrelated to any known pathological condition that can form amyloid fibrils in vitro [43]. Aggregated species obtained from this protein have been found to be cytotoxic when added to cell cultures [44]. We have previously shown that the α-spectrin-SH3 (SPC-SH3) domain, which shares the same fold and 24% sequence identity with PI3-SH3, is a soluble protein that does not form amyloid fibrils under any conditions tested [45]. Nevertheless, a recent work found that the N47A mutation at the distal loop induces the formation of amyloid fibrils [46]. In contrast, the mutation of residue 47 to Gly does not promote aggregation (Ventura, S., unpublished results). According to AGGRESCAN a new HS occurs in the amyloidogenic mutant relative to both the wild type and N47G species, which could be responsible for its increased aggregation abilities (Figure 3).
Analysis of protein datasets
Besides analyzing the theoretical aggregation properties of single molecules and their individual mutants, AGGRESCAN is also able to deal simultaneously with a large number of sequences. This ability can be specially useful to compare the global aggregation properties of different protein sets and may help to delineate general rules underlying the relationship between the primary structure of proteins and peptides and their specific in vivo and in vitro depositional properties. With this aim we studied the correlation between the structural/aggregational features of 5 different groups of proteins and the predictions provided by AGGRESCAN. These datasets were: 1) natively globular proteins (160 proteins) (from SCOP, the ASTRAL40 set); 2) natively intrinsically unstructured proteins (51 proteins); 3) proteins which are soluble when overexpressed in bacteria (38 proteins); 4) proteins forming inclusion bodies when overexpressed in bacteria (121 proteins) and 5) amyloidogenic proteins (57 proteins) (see additional file 4).
When average AGGRESCAN output values are calculated and subsequently compared between data sets, it appears that the different protein groups can be individualized (Table 5), providing insight into the sequential determinants of protein aggregation and solubility. In this way, intrinsically unstructured proteins (IUP) clearly present the lowest output values of all datasets, in correlation with the general observation that unstructured proteins are usually resistant to aggregation and remain soluble after heat-treatment of the cells. Natively unfolded proteins exhibit a Na4vSS value 7 times lower than that corresponding to the set of globular proteins from SCOP. Also, the normalized number of HS (NnHS) or the area over the threshold (AAT) and total HS area (THSA) are around 2 times higher in globular proteins than in IUP, showing that, in agreement with other automated analyses [47], the number of aggregation-prone sequence stretches is lower in IUP than in structured proteins. This result may reflect a negative natural selection against aggregation promoting residues and regions in IUP, where any HS will be exposed to solvent and accessible for the establishment of inter-molecular contacts that may finally lead to the build-up of aggregates. For the same reason, nature is likely to have provided globular proteins with a stable native conformation in which aggregation-prone sequences are buried in the inner hydrophobic core or involved in intra-molecular interactions [13, 18]. This appears to be a successful evolutive strategy to avoid deposition, since few proteins aggregate from their folded state. Hence, amyloidogenic mutations in globular proteins usually result in destabilization of the native state, permitting exposure of natively hidden HS.
It has been recently shown that proteins that form inclusion bodies (IB) upon recombinant overexpression in E. coli and proteins that form amyloids in vivo and/or in vitro share a good number of structural and functional features, including high purity of the aggregates, enrichment in beta-sheet structure, amyloid-tropic dye binding or enhanced proteolytic resistance [3]. Comparison of the two protein sets in search for similar trends in the predictions showed that, unexpectedly, the AGGRESCAN values for amyloid forming proteins are closer to those for IUP than for any other of the analysed datasets. Amyloid proteins have a lower Na4vSS and less HS than proteins in the IB or globular SCOP dataset (Figure 4). In contrast, the HSs in amyloid proteins comprise an area similar to those in IB or globular proteins, which is, however, significantly higher than the average HS area in IUP. These results suggests that, globally, the sequences of amyloidogenic proteins, like those of IUPs, have a low aggregation propensity, although the existence of specific aggregation-prone regions, absent or minor in IUPs, in a context in which they can act as specific and obligatory nucleation points from which the fibrillar structure could be expanded, finally results in highly ordered aggregates (Figure 4). This would explain why point mutations in the HSs of amyloidogenic proteins have usually a huge impact in their solubility, as they would modify the properties of one of the few points in the sequence that can promote and/or modulate aggregation. In contrast, the paradoxically higher-ranking global aggregation propensity of IB protein sequences is likely to indicate that here HS would play a less important role, since aggregation can also occur non specifically from many regions in the protein sequence. This would result in less structured deposits, and would also explain the rather moderate role of point mutations in IB aggregate formation. In other words, a given HS would promote specific amyloid formation in a low aggregating background, as its aggregation tendency outstands from the rest of the sequence. Conversely, the same HS needs to compete with the rest of the sequence to nucleate aggregation in a highly aggregating context (Figure 5). For the same reason unstructured aggregation is usually a much faster event than amyloidogenesis. Recent theoretical and experimental data support this view by showing that prevention of aggregation does not necessarily mean that amyloid fibril formation is abolished and vice versa [48]. This indicates that, despite the fact that aggregates and amyloid fibrils share many features, and the protein regions involved in their formation presumably intersect, they probably differ in the number and specificity of intermolecular contacts involved in the nucleation and stabilization of both types of polypeptide associations.
"Hot spots" distribution in different protein groups. Distribution of the number of "hot spots" relative to sequence length in the following protein datasets: natively globular proteins, intrinsically unstructured proteins, amyloidogenic proteins, soluble proteins when overexpressed in bacteria and proteins forming inclusion bodies when overexpressed in bacteria.
Modulation of hot spot nucleation specificity by global aggregation propensity. The black solid line represents a standard amyloidogenic protein aggregation profile, with only one "hot spot" and low global aggregation propensity. The pink discontinuous line corresponds to a typical aggregation profile from an inclusion-body-forming protein, with many "hot spots" and high global aggregation propensity. The horizontal lines represent the aggregation-propensity average thresholds for each sequence. The coloured regions indicate the area of each "hot spot" over the aggregation propensity threshold. It is proposed that a higher area over the threshold promotes a more specific aggregation reaction, resulting in highly ordered deposits.
Recombinant protein production is an essential tool for the biotechnological industry and supports expanding areas of basic and biomedical research, including structural genomics and proteomics. The solubility of proteins expressed in bacteria under mass-production conditions is of major concern, since many recombinant polypeptides produced in bacteria accumulate as insoluble, often refractile, aggregates known as inclusion bodies (IBs) [49], excluding many biotechnologically relevant protein species from the market due to economically inconvenient yields. To date, the solubility of a given gene product has not been anticipated before gene expression. The comparison between the AGGRESCAN output values for proteins shown to be soluble under overexpression conditions in E. coli and those forming inclusion bodies shows that they share a similar number of HSs per 100 residues (NnHS), an expected output when considering that most proteins in both datasets are globular. However, IB-forming proteins have higher Na4vSS values than soluble proteins, suggesting that soluble proteins have, on average, a lower intrinsic aggregation tendency than IB-forming species, which may determine, at least partially, their relative behaviour upon overexpression. Overall, the predicted aggregation of proteins in the SCOP database is intermediate between that of soluble and insoluble proteins, suggesting that, in agreement with experimental observations, only a part of them would remain in the soluble cell fraction upon recombinant production. Although AGGRESCAN is able to catch the average trends in the aggregation of IBs and soluble protein groups, the individual outputs for proteins from both groups overlap significantly, making the prediction of the recombinant behaviour of a given sequence difficult in its present form. Besides, aggregation during recombinant production is the net result of several extrinsic and intrinsic factors, their relative importance depending on the protein and expression contexts.
Conclusion
The software and web interface developed in the present study allow an easy and accurate identification and ranking of aggregation-prone regions in polypeptides. AGGRESCAN is also able to anticipate the effect of genetic or artificially introduced sequence changes on the aggregation properties of polypeptides. In addition to the investigation of the role of the primary sequence on protein aggregation and protein solubility, the algorithm can be used in the design of strategies for the treatment of amyloidogenesis, by targeting therapies to those regions in the polypeptide chain whose aggregation propensities outstand from the rest, provided that they are or become exposed to solvent in the disease-related protein conformation. The surprising observation that the aggregation propensities of amyloid sequences tend to be low, suggests that blocking the "hot spots" of aggregation in these proteins, either chemically or by mutation, may have a huge impact on their solubility. Interestingly enough, protein-protein interactions are often mediated through an energetic hot spot [50] which comprises few interface residues that contribute to most of the binding energy; identification and blocking of those sequence stretches has been suggested as an strategy to modulate protein interactions [51]. The ability of AGGRESCAN to analyze simultaneously the aggregation properties of large sets of protein sequences might be important for protein production in large-scale structural initiatives, for the analysis of the distribution of aggregation-prone regions in complete genomes or for evolutive studies, since it is likely that natural protein sequences have evolved in part to code for avoidance of aggregation.
Availability and requirements
Project name: AGGRESCAN
Project home page: http://bioinf.uab.es/aggrescan/
Operating system(s): Platform independent
Programming language: a computing core coded in C and a front end written in a combination of html and perl cgi.
Other requirements: a web browser, such as Internet Explorer, Safari, or Firefox.
Any restrictions to use by non-academics: Incorporation into commercial products restricted.
References
Fink AL: Protein aggregation: folding aggregates, inclusion bodies and amyloid. Fold Des 1998, 3: R9 -23. 10.1016/S1359-0278(98)00002-9
Smith A: protein misfolding. Nature 2003, 426: 883 -8883. 10.1038/426883a
Ventura S, Villaverde A: Protein quality in bacterial inclusion bodies. Trends Biotechnol 2006, 24(4):179–185. 10.1016/j.tibtech.2006.02.007
Treuheit MJ, Kosky AA, Brems DN: Inverse relationship of protein concentration and aggregation. Pharm Res 2002, 19(4):511–516. 10.1023/A:1015108115452
Dobson CM: Protein-misfolding diseases: Getting out of shape. Nature 2002, 418: 729 -7730. 10.1038/418729a
Cohen FE, Kelly JW: Therapeutic approaches to protein-misfolding diseases. Nature 2003, 426: 905 -9909. 10.1038/nature02265
Rochet JC, Lansbury PT: Amyloid fibrillogenesis: themes and variations. Curr Opin Struct Biol 2000, 10: 60 -668. 10.1016/S0959-440X(99)00049-4
Stefani M, Dobson CM: Protein aggregation and aggregate toxicity: new insights into protein folding, misfolding diseases and biological evolution. J Mol Med 2003, 81(11):678–699. 10.1007/s00109-003-0464-5
Ivanova MI, Sawaya MR, Gingery M, Attinger A, Eisenberg D: An amyloid-forming segment of {beta}2-microglobulin suggests a molecular model for the fibril. PNAS 2004, 101(29):10584–10589. 10.1073/pnas.0403756101
Ventura S, Zurdo J, Narayanan S, Parreno M, Mangues R, Reif B, Chiti F, Giannoni E, Dobson CM, Aviles FX, Serrano L: Short amino acid stretches can mediate amyloid formation in globular proteins: the Src homology 3 (SH3) case. Proc Natl Acad Sci U S A 2004, 101: 7258 -77263. 10.1073/pnas.0308249101
Chiti F, Dobson CM: Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem 2006, 75: 333–366. 10.1146/annurev.biochem.75.101304.123901
de Groot NS, Aviles FX, Vendrell J, Ventura S: Mutagenesis of the central hydrophobic cluster in Abeta42 Alzheimer's peptide. Side-chain properties correlate with aggregation propensities. Febs J 2006, 273(3):658–668. 10.1111/j.1742-4658.2005.05102.x
de Groot N, Pallares I, Aviles F, Vendrell J, Ventura S: Prediction of "hot spots" of aggregation in disease-linked polypeptides. BMC Structural Biology 2005, 5(1):18. 10.1186/1472-6807-5-18
Chiti F, Stefani M, Taddei N, Ramponi G, Dobson CM: Rationalization of the effects of mutations on peptide and protein aggregation rates. Nature 2003, 424(6950):805–808. 10.1038/nature01891
Williams AD, Portelius E, Kheterpal I, Guo JT, Cook KD, Xu Y, Wetzel R: Mapping abeta amyloid fibril secondary structure using scanning proline mutagenesis. J Mol Biol 2004, 335(3):833–842. 10.1016/j.jmb.2003.11.008
Chiti F, Webster P, Taddei N, Clark A, Stefani M, Ramponi G, Dobson CM: Designing conditions for in vitro formation of amyloid protofilaments and fibrils. Proc Natl Acad Sci U S A 1999, 96(7):3590–3594. 10.1073/pnas.96.7.3590
Chiti F, Calamai M, Taddei N, Stefani M, Ramponi G, Dobson CM: Studies of the aggregation of mutant proteins in vitro provide insights into the genetics of amyloid diseases. Proc Natl Acad Sci U S A 2002, 99 Suppl 4: 16419–16426. 10.1073/pnas.212527999
Rojas Quijano FA, Morrow D, Wise BM, Brancia FL, Goux WJ: Prediction of nucleating sequences from amyloidogenic propensities of tau-related peptides. Biochemistry 2006, 45(14):4638–4652. 10.1021/bi052226q
Ivanova MI, Thompson MJ, Eisenberg D: A systematic screen of beta(2)-microglobulin and insulin for amyloid-like segments. Proc Natl Acad Sci U S A 2006, 103(11):4079–4082. 10.1073/pnas.0511298103
Fernandez-Escamilla AM, Rousseau F, Schymkowitz J, Serrano L: Prediction of sequence-dependent and mutational effects on the aggregation of peptides and proteins. Nat Biotechnol 2004, 22: 1302 -11306. 10.1038/nbt1012
DuBay KF, Pawar AP, Chiti F, Zurdo J, Dobson CM, Vendruscolo M: Prediction of the absolute aggregation rates of amyloidogenic polypeptide chains. J Mol Biol 2004, 341(5):1317–1326. 10.1016/j.jmb.2004.06.043
Tartaglia GG, Cavalli A, Pellarin R, Caflisch A: Prediction of aggregation rate and aggregation-prone segments in polypeptide sequences. Protein Sci 2005, 14(10):2723–2734. 10.1110/ps.051471205
Idicula-Thomas S, Balaji PV: Understanding the relationship between the primary structure of proteins and their amyloidogenic propensity: clues from inclusion body formation. Protein Eng Des Sel 2005, 18(4):175–180. 10.1093/protein/gzi022
Johansson J, Weaver TE, Tjernberg LO: Proteolytic generation and aggregation of peptides from transmembrane regions: lung surfactant protein C and amyloid beta-peptide. Cell Mol Life Sci 2004, 61(3):326–335. 10.1007/s00018-003-3274-6
Westermark P, Johnson KH, O'Brien TD, Betsholtz C: Islet amyloid polypeptide--a novel controversy in diabetes research. Diabetologia 1992, 35(4):297–303. 10.1007/BF00401195
Margittai M, Langen R: Template-assisted filament growth by parallel stacking of tau. Proc Natl Acad Sci U S A 2004, 101(28):10278–10283. 10.1073/pnas.0401911101
Selkoe DJ: Cell biology of protein misfolding: the examples of Alzheimer's and Parkinson's diseases. Nat Cell Biol 2004, 6(11):1054–1061. 10.1038/ncb1104-1054
Nelson R, Eisenberg D: Structural models of amyloid-like fibrils. Adv Protein Chem 2006, 73: 235–282.
Pawar AP, Dubay KF, Zurdo J, Chiti F, Vendruscolo M, Dobson CM: Prediction of "aggregation-prone" and "aggregation-susceptible" regions in proteins associated with neurodegenerative diseases. J Mol Biol 2005, 350(2):379–392. 10.1016/j.jmb.2005.04.016
Galzitskaya OV, Garbuzynskiy SO, Lobanov MY: Prediction of amyloidogenic and disordered regions in protein chains. PLoS Comput Biol 2006, 2(12):e177. 10.1371/journal.pcbi.0020177
Thompson MJ, Sievers SA, Karanicolas J, Ivanova MI, Baker D, Eisenberg D: The 3D profile method for identifying fibril-forming segments of proteins. Proc Natl Acad Sci U S A 2006, 103(11):4074–4078. 10.1073/pnas.0511295103
Lopez De La Paz M, Goldie K, Zurdo J, Lacroix E, Dobson CM, Hoenger A, Serrano L: De novo designed peptide-based amyloid fibrils. Proc Natl Acad Sci U S A 2002, 99(25):16052–16057. 10.1073/pnas.252340199
Fowler SB, Poon S, Muff R, Chiti F, Dobson CM, Zurdo J: Rational design of aggregation-resistant bioactive peptides: reengineering human calcitonin. Proc Natl Acad Sci U S A 2005, 102(29):10105–10110. 10.1073/pnas.0501215102
Esler WP, Stimson ER, Ghilardi JR, Lu YA, Felix AM, Vinters HV, Mantyh PW, Lee JP, Maggio JE: Point substitution in the central hydrophobic cluster of a human beta-amyloid congener disrupts peptide folding and abolishes plaque competence. Biochemistry 1996, 35: 13914 -13921. 10.1021/bi961302+
Lambermon MH, Rappaport RV, McLaurin J: Biophysical characterization of longer forms of amyloid beta peptides: possible contribution to flocculent plaque formation. J Neurochem 2005, 95(6):1667–1676. 10.1111/j.1471-4159.2005.03497.x
Gamblin TC, Berry RW, Binder LI: Tau polymerization: role of the amino terminus. Biochemistry 2003, 42(7):2252–2257. 10.1021/bi0272510
Barghorn S, Mandelkow E: Toward a unified scheme for the aggregation of tau into Alzheimer paired helical filaments. Biochemistry 2002, 41(50):14885–14896. 10.1021/bi026469j
Li L, von Bergen M, Mandelkow EM, Mandelkow E: Structure, stability, and aggregation of paired helical filaments from tau protein and FTDP-17 mutants probed by tryptophan scanning mutagenesis. J Biol Chem 2002, 277(44):41390–41400. 10.1074/jbc.M206334200
Yao TM, Tomoo K, Ishida T, Hasegawa H, Sasaki M, Taniguchi T: Aggregation analysis of the microtubule binding domain in tau protein by spectroscopic methods. J Biochem (Tokyo) 2003, 134(1):91–99.
Rabzelj S, Turk V, Zerovnik E: In vitro study of stability and amyloid-fibril formation of two mutants of human stefin B (cystatin B) occurring in patients with EPM1. Protein Sci 2005, 14(10):2713–2722. 10.1110/ps.051609705
Delibas A, Oner A, Balci B, Demircin G, Bulbul M, Bek K, Erdogan O, Baysun S, Yilmaz E: Genetic risk factors of amyloidogenesis in familial Mediterranean fever. Am J Nephrol 2005, 25(5):434–440. 10.1159/000087824
Jimenez JL, Guijarro JI, Orlova E, Zurdo J, Dobson CM, Sunde M, Saibil HR: Cryo-electron microscopy structure of an SH3 amyloid fibril and model of the molecular packing. Embo J 1999, 18(4):815–821. 10.1093/emboj/18.4.815
Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo J, Taddei N, Ramponi G, Dobson CM, Stefani M: Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 2002, 416(6880):507–511. 10.1038/416507a
Ventura S, Lacroix E, Serrano L: Insights into the origin of the tendency of the PI3-SH3 domain to form amyloid fibrils. J Mol Biol 2002, 322: 1147 -11458. 10.1016/S0022-2836(02)00783-0
Morel B, Casares S, Conejero-Lara F: A single mutation induces amyloid aggregation in the alpha-spectrin SH3 domain: analysis of the early stages of fibril formation. J Mol Biol 2006, 356(2):453–468. 10.1016/j.jmb.2005.11.062
Linding R, Schymkowitz J, Rousseau F, Diella F, Serrano L: A comparative study of the relationship between protein structure and beta-aggregation in globular and intrinsically disordered proteins. J Mol Biol 2004, 342(1):345–353. 10.1016/j.jmb.2004.06.088
Rousseau F, Schymkowitz J, Serrano L: Protein aggregation and amyloidosis: confusion of the kinds? Curr Opin Struct Biol 2006, 16(1):118–126. 10.1016/j.sbi.2006.01.011
Villaverde A, Carrio MM: Protein aggregation in recombinant bacteria: biological role of inclusion bodies. Biotechnol Lett 2003, 25(17):1385–1395. 10.1023/A:1025024104862
Clackson T, Wells JA: A hot spot of binding energy in a hormone-receptor interface. Science 1995, 267(5196):383–386. 10.1126/science.7529940
Keskin O, Ma B, Nussinov R: Hot regions in protein--protein interactions: the organization and contribution of structurally conserved hot spot residues. J Mol Biol 2005, 345(5):1281–1294. 10.1016/j.jmb.2004.10.077
El-Agnaf O, Gibson G, Lee M, Wright A, Austen BM: Properties of neurotoxic peptides related to the Bri gene. Protein Pept Lett 2004, 11(3):207–212. 10.2174/0929866043407156
El-Agnaf OM, Nagala S, Patel BP, Austen BM: Non-fibrillar oligomeric species of the amyloid ABri peptide, implicated in familial British dementia, are more potent at inducing apoptotic cell death than protofibrils or mature fibrils. J Mol Biol 2001, 310(1):157–168. 10.1006/jmbi.2001.4743
Goedert M: Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci 2001, 2(7):492–501. 10.1038/35081564
Bodles AM, Guthrie DJ, Greer B, Irvine GB: Identification of the region of non-Abeta component (NAC) of Alzheimer's disease amyloid responsible for its aggregation and toxicity. J Neurochem 2001, 78(2):384–395. 10.1046/j.1471-4159.2001.00408.x
Miake H, Mizusawa H, Iwatsubo T, Hasegawa M: Biochemical characterization of the core structure of alpha-synuclein filaments. J Biol Chem 2002, 277(21):19213–19219. 10.1074/jbc.M110551200
Kallijarvi J, Haltia M, Baumann MH: Amphoterin includes a sequence motif which is homologous to the Alzheimer's beta-amyloid peptide (Abeta), forms amyloid fibrils in vitro, and binds avidly to Abeta. Biochemistry 2001, 40(34):10032–10037. 10.1021/bi002095n
Morimoto A, Irie K, Murakami K, Masuda Y, Ohigashi H, Nagao M, Fukuda H, Shimizu T, Shirasawa T: Analysis of the secondary structure of beta-amyloid (Abeta42) fibrils by systematic proline replacement. J Biol Chem 2004, 279(50):52781–52788. 10.1074/jbc.M406262200
Nichols WC, Dwulet FE, Liepnieks J, Benson MD: Variant apolipoprotein AI as a major constituent of a human hereditary amyloid. Biochem Biophys Res Commun 1988, 156(2):762–768. 10.1016/S0006-291X(88)80909-4
Wilson LM, Mok YF, Binger KJ, Griffin MD, Mertens HD, Lin F, Wade JD, Gooley PR, Howlett GJ: A Structural Core Within Apolipoprotein C-II Amyloid Fibrils Identified Using Hydrogen Exchange and Proteolysis. J Mol Biol 2007, 366(5):1639–51. 10.1016/j.jmb.2006.12.040
Hasegawa K, Ohhashi Y, Yamaguchi I, Takahashi N, Tsutsumi S, Goto Y, Gejyo F, Naiki H: Amyloidogenic synthetic peptides of beta2-microglobulin--a role of the disulfide bond. Biochem Biophys Res Commun 2003, 304(1):101–106. 10.1016/S0006-291X(03)00543-6
Jones S, Manning J, Kad NM, Radford SE: Amyloid-forming peptides from beta2-microglobulin-Insights into the mechanism of fibril formation in vitro. J Mol Biol 2003, 325(2):249–257. 10.1016/S0022-2836(02)01227-5
Tamburro AM, Pepe A, Bochicchio B, Quaglino D, Ronchetti IP: Supramolecular amyloid-like assembly of the polypeptide sequence coded by exon 30 of human tropoelastin. J Biol Chem 2005, 280(4):2682–2690. 10.1074/jbc.M411617200
Hamidi Asl L, Liepnieks JJ, Uemichi T, Rebibou JM, Justrabo E, Droz D, Mousson C, Chalopin JM, Benson MD, Delpech M, Grateau G: Renal amyloidosis with a frame shift mutation in fibrinogen aalpha-chain gene producing a novel amyloid protein. Blood 1997, 90(12):4799–4805.
Liu W, Crocker E, Zhang W, Elliott JI, Luy B, Li H, Aimoto S, Smith SO: Structural role of glycine in amyloid fibrils formed from transmembrane alpha-helices. Biochemistry 2005, 44(9):3591–3597. 10.1021/bi047827g
Jimenez JL, Nettleton EJ, Bouchard M, Robinson CV, Dobson CM, Saibil HR: The protofilament structure of insulin amyloid fibrils. Proc Natl Acad Sci U S A 2002, 99(14):9196–9201. 10.1073/pnas.142459399
Scrocchi LA, Ha K, Chen Y, Wu L, Wang F, Fraser PE: Identification of minimal peptide sequences in the (8–20) domain of human islet amyloid polypeptide involved in fibrillogenesis. J Struct Biol 2003, 141(3):218–227. 10.1016/S1047-8477(02)00630-5
Azriel R, Gazit E: Analysis of the minimal amyloid-forming fragment of the islet amyloid polypeptide. An experimental support for the key role of the phenylalanine residue in amyloid formation. J Biol Chem 2001, 276(36):34156–34161. 10.1074/jbc.M102883200
Krebs MR, Wilkins DK, Chung EW, Pitkeathly MC, Chamberlain AK, Zurdo J, Robinson CV, Dobson CM: Formation and seeding of amyloid fibrils from wild-type hen lysozyme and a peptide fragment from the beta-domain. J Mol Biol 2000, 300(3):541–549. 10.1006/jmbi.2000.3862
Frare E, Polverino De Laureto P, Zurdo J, Dobson CM, Fontana A: A highly amyloidogenic region of hen lysozyme. J Mol Biol 2004, 340(5):1153–1165. 10.1016/j.jmb.2004.05.056
Reches M, Gazit E: Amyloidogenic hexapeptide fragment of medin: homology to functional islet amyloid polypeptide fragments. Amyloid 2004, 11(2):81–89.
Fandrich M, Forge V, Buder K, Kittler M, Dobson CM, Diekmann S: Myoglobin forms amyloid fibrils by association of unfolded polypeptide segments. Proc Natl Acad Sci U S A 2003, 100(26):15463–15468. 10.1073/pnas.0303758100
Tagliavini F, Prelli F, Verga L, Giaccone G, Sarma R, Gorevic P, Ghetti B, Passerini F, Ghibaudi E, Forloni G, et al.: Synthetic peptides homologous to prion protein residues 106–147 form amyloid-like fibrils in vitro. Proc Natl Acad Sci U S A 1993, 90(20):9678–9682. 10.1073/pnas.90.20.9678
Hinton DR, Polk RK, Linse KD, Weiss MH, Kovacs K, Garner JA: Characterization of spherical amyloid protein from a prolactin-producing pituitary adenoma. Acta Neuropathol (Berl) 1997, 93(1):43–49. 10.1007/s004010050581
Westermark GT, Engstrom U, Westermark P: The N-terminal segment of protein AA determines its fibrillogenic property. Biochem Biophys Res Commun 1992, 182(1):27–33. 10.1016/S0006-291X(05)80107-X
Jarvis JA, Kirkpatrick A, Craik DJ: 1H NMR analysis of fibril-forming peptide fragments of transthyretin. Int J Pept Protein Res 1994, 44(4):388–398.
Jaroniec CP, MacPhee CE, Bajaj VS, McMahon MT, Dobson CM, Griffin RG: High-resolution molecular structure of a peptide in an amyloid fibril determined by magic angle spinning NMR spectroscopy. Proc Natl Acad Sci U S A 2004, 101(3):711–716. 10.1073/pnas.0304849101
Petkova AT, Ishii Y, Balbach JJ, Antzutkin ON, Leapman RD, Delaglio F, Tycko R: A structural model for Alzheimer's beta -amyloid fibrils based on experimental constraints from solid state NMR. Proc Natl Acad Sci U S A 2002, 99(26):16742–16747. 10.1073/pnas.262663499
Kajava AV, Aebi U, Steven AC: The parallel superpleated beta-structure as a model for amyloid fibrils of human amylin. J Mol Biol 2005, 348(2):247–252. 10.1016/j.jmb.2005.02.029
Ritter C, Maddelein ML, Siemer AB, Luhrs T, Ernst M, Meier BH, Saupe SJ, Riek R: Correlation of structural elements and infectivity of the HET-s prion. Nature 2005, 435(7043):844–848. 10.1038/nature03793
Lim KH, Nguyen TN, Damo SM, Mazur T, Ball HL, Prusiner SB, Pines A, Wemmer DE: Solid-state NMR structural studies of the fibril form of a mutant mouse prion peptide PrP89–143(P101L). Solid State Nucl Magn Reson 2006, 29(1–3):183–190. 10.1016/j.ssnmr.2005.09.017
Iwata K, Fujiwara T, Matsuki Y, Akutsu H, Takahashi S, Naiki H, Goto Y: 3D structure of amyloid protofilaments of beta2-microglobulin fragment probed by solid-state NMR. Proc Natl Acad Sci U S A 2006, 103(48):18119–18124. 10.1073/pnas.0607180103
Jaroniec CP, MacPhee CE, Astrof NS, Dobson CM, Griffin RG: Molecular conformation of a peptide fragment of transthyretin in an amyloid fibril. Proc Natl Acad Sci U S A 2002, 99(26):16748–16753. 10.1073/pnas.252625999
Yamamoto N, Hasegawa K, Matsuzaki K, Naiki H, Yanagisawa K: Environment- and mutation-dependent aggregation behavior of Alzheimer amyloid beta-protein. J Neurochem 2004, 90(1):62–69. 10.1111/j.1471-4159.2004.02459.x
Cannon MJ, Williams AD, Wetzel R, Myszka DG: Kinetic analysis of beta-amyloid fibril elongation. Anal Biochem 2004, 328(1):67–75. 10.1016/j.ab.2004.01.014
Van Nostrand WE, Melchor JP, Cho HS, Greenberg SM, Rebeck GW: Pathogenic effects of D23N Iowa mutant amyloid beta -protein. J Biol Chem 2001, 276(35):32860–32866. 10.1074/jbc.M104135200
Wurth C, Guimard NK, Hecht MH: Mutations that reduce aggregation of the Alzheimer's Abeta42 peptide: an unbiased search for the sequence determinants of Abeta amyloidogenesis. J Mol Biol 2002, 319(5):1279–1290. 10.1016/S0022-2836(02)00399-6
Jarrett JT, Berger EP, Lansbury PT Jr.: The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer's disease. Biochemistry 1993, 32(18):4693–4697. 10.1021/bi00069a001
Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL: Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer's disease. Proc Natl Acad Sci U S A 2003, 100(17):10032–10037. 10.1073/pnas.1630428100
Barghorn S, Zheng-Fischhofer Q, Ackmann M, Biernat J, von Bergen M, Mandelkow EM, Mandelkow E: Structure, microtubule interactions, and paired helical filament aggregation by tau mutants of frontotemporal dementias. Biochemistry 2000, 39(38):11714–11721. 10.1021/bi000850r
Rosso SM, van Herpen E, Deelen W, Kamphorst W, Severijnen LA, Willemsen R, Ravid R, Niermeijer MF, Dooijes D, Smith MJ, Goedert M, Heutink P, van Swieten JC: A novel tau mutation, S320F, causes a tauopathy with inclusions similar to those in Pick's disease. Ann Neurol 2002, 51(3):373–376. 10.1002/ana.10140
Choi W, Zibaee S, Jakes R, Serpell LC, Davletov B, Crowther RA, Goedert M: Mutation E46K increases phospholipid binding and assembly into filaments of human alpha-synuclein. FEBS Lett 2004, 576(3):363–368. 10.1016/j.febslet.2004.09.038
Giasson BI, Murray IV, Trojanowski JQ, Lee VM: A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J Biol Chem 2001, 276(4):2380–2386. 10.1074/jbc.M008919200
Green J, Goldsbury C, Mini T, Sunderji S, Frey P, Kistler J, Cooper G, Aebi U: Full-length rat amylin forms fibrils following substitution of single residues from human amylin. J Mol Biol 2003, 326(4):1147–1156. 10.1016/S0022-2836(02)01377-3
Sakagashira S, Sanke T, Hanabusa T, Shimomura H, Ohagi S, Kumagaye KY, Nakajima K, Nanjo K: Missense mutation of amylin gene (S20G) in Japanese NIDDM patients. Diabetes 1996, 45(9):1279–1281. 10.2337/diabetes.45.9.1279
Porte D Jr., Kahn SE: Hyperproinsulinemia and amyloid in NIDDM. Clues to etiology of islet beta-cell dysfunction? Diabetes 1989, 38(11):1333–1336. 10.2337/diabetes.38.11.1333
Salmona M, Malesani P, De Gioia L, Gorla S, Bruschi M, Molinari A, Della Vedova F, Pedrotti B, Marrari MA, Awan T, Bugiani O, Forloni G, Tagliavini F: Molecular determinants of the physicochemical properties of a critical prion protein region comprising residues 106–126. Biochem J 1999, 342 ( Pt 1): 207–214. 10.1042/0264-6021:3420207
Thompson AJ, Barnham KJ, Norton RS, Barrow CJ: The Val-210-Ile pathogenic Creutzfeldt-Jakob disease mutation increases both the helical and aggregation propensities of a sequence corresponding to helix-3 of PrP(C). Biochim Biophys Acta 2001, 1544(1–2):242–254.
Acknowledgements
This work was supported by grants BIO2004-05879 and BIO2003-02848 (Ministerio de Educación y Ciencia, Spain) and SGR2005-00037 and SGR2005-01037 (Generalitat de Catalunya, Spain). NS is recipient of a fellowship from the Spanish Ministerio de Educación y Ciencia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
OCS implemented the software, NSG analyzed and prepared the final data and figures. FXA and JV contributed to data interpretation and manuscript redaction. XD directed the implementation of the software and contributed to manuscript redaction. SV directed the work and prepared the manuscript. All authors read and approved the final manuscript.
Oscar Conchillo-Solé, Natalia S de Groot contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Conchillo-Solé, O., de Groot, N.S., Avilés, F.X. et al. AGGRESCAN: a server for the prediction and evaluation of "hot spots" of aggregation in polypeptides. BMC Bioinformatics 8, 65 (2007). https://doi.org/10.1186/1471-2105-8-65
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2105-8-65