- Correspondence
- Open access
- Published:
Reporting guidelines for modelling studies
BMC Medical Research Methodology volume 12, Article number: 168 (2012)
Abstract
Background
Modelling studies are used widely to help inform decisions about health care and policy and their use is increasing. However, in order for modelling to gain strength as a tool for health policy, it is critical that key model factors are transparent so that users of models can have a clear understanding of the model and its limitations.Reporting guidelines are evidence-based tools that specify minimum criteria for authors to report their research such that readers can both critically appraise and interpret study findings. This study was conducted to determine whether there is an unmet need for population modelling reporting guidelines.
Methods
We conducted a review of the literature to identify: 1) guidance for reporting population modelling studies; and, 2) evidence on the quality of reporting of population modelling studies. Guidance for reporting was analysed using a thematic approach and the data was summarised as frequencies. Evidence on the quality of reporting was reviewed and summarized descriptively.
Results
There were no guidelines that specifically addressed the reporting of population modelling studies. We identified a number of reporting guidelines for economic evaluation studies, some of which had sections that were relevant population modelling studies. Amongst seven relevant records, we identified 69 quality criteria that have distinct reporting characteristics. We identified two papers that addressed reporting practices of modelling studies. Overall, with the exception of describing the data used for calibration, there was little consistency in reporting.
Conclusions
While numerous guidelines exist for developing and evaluating health technology assessment and economic evaluation models, which by extension could be applicable to population modelling studies, there is variation in their comprehensiveness and in the consistency of reporting these methods. Population modelling studies may be an area which would benefit from the development of a reporting guideline.
Introduction
Modelling studies are used widely to help inform decisions about health care and policy and their use is increasing [1, 2]. A model is “an analytical methodology that accounts for events over time and across populations, that is based on data drawn from primary or secondary sources…” and in the context of health care-evaluation “…whose purpose is to estimate the effects of an intervention on valued health consequences and costs” [3]. Its value lies not only in its results, but also in its ability to reveal the connections between its data and assumptions and model outputs [3]. But, as pointed out by Garrison, models don’t have to be mathematically sophisticated to be hard to follow [4]. For these reasons, a model should not be a “black box” for the end-user but be as transparent as possible [3].
To address the problem of poorly reported research, multiple reporting guidelines have been developed and validated for use with a number of study designs. Reporting guidelines are evidence-based tools that employ expert consensus to specify minimum criteria for authors to report their research such that readers can both critically appraise and interpret study findings [5, 6]. The EQUATOR Network, an international initiative whose aim is to improve the reliability of medical research by promoting transparent and accurate reporting of research studies, indexes more than 100 reporting guidelines on their Web site (http://www.equator-network.org).
The growth in the number and range of reporting guidelines has prompted guidance on how to develop one using a well-structured development process [6]. This study addresses the needs assessment-that is, to determine whether there is a need for population modelling reporting guidelines. More specifically, the objectives of our study were: to locate and assess any existing reporting guidelines for population modelling studies; to identify key quality criteria for the reporting of population modelling studies; and to determine if and how these criteria are being reported in the literature.
Methods
We began this process with a search of the MEDLINE electronic database (MEDLINE (1950 – February 2011) via Ovid. Our electronic search strategy (see appendix), developed in consultation with a library scientist, was pragmatically designed to avoid being overwhelmed with irrelevant records. We hand-searched the reference lists and used the related articles feature in PubMED for all papers meeting our eligibility criteria. In addition, we reviewed relevant textbooks and Web sites. One reviewer screened the titles and abstracts of all unique citations to identify papers that met our inclusion criteria—that is, English language papers that provided explicit guidance on the reporting of population modelling studies or provided evidence on the quality of reporting of population modelling studies in the health science literature. The full-text report of each record passing title/abstract screening was retrieved and reviewed by the research team and its inclusion/exclusion status was established.
For records that provided explicit guidance on reporting of population modelling studies, the list of criteria identified was analysed using a thematic approach and the data was summarised as frequencies. For those papers that presented evidence on the quality of reporting of population modelling studies, we identified the aspects of reporting that were assessed and summarised the results descriptively.
Results and discussion
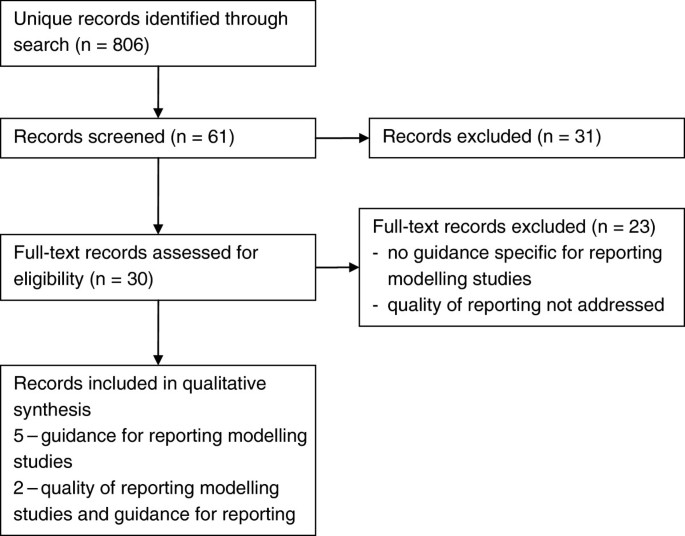
We identified 806 unique records through our search strategy, 30 full-text articles were reviewed to determine eligibility (Figure 1).
Existence of guidelines for modelling studies
There were no guidelines that specifically addressed the reporting of population modelling studies. However, there were a number of reporting guidelines for economic evaluation studies: one of which was related to modelling [7] and one included a section which focused on the generalisability of modelling studies [8]. Additionally, we identified one paper that provided reporting guidance for a specific aspect of simulation modelling methodology – calibration [9].
Numerous guidelines have been published defining good practice for the conduct of economic evaluations in general and model-based evaluations in particular. We identified two papers that provided guidance for assessing the quality of decision-analytic modelling studies [3, 10] and one paper that provided guidance for assessing validation of population-based disease simulation models [2].
Identification of key reporting items
Amongst the relevant records that were analysed, we identified 69 quality criteria that have distinct reporting characteristics (Table 1).
We identified 22 items relating to the structure of the model and broadly classified them into 10 domains: 1) statement of decision problem/objective; 2) statement of scope/perspective; 3) rationale for structure; 4) structural assumptions; 5) strategies/comparators; 6) model type; 7) time horizon; 8) disease states/pathways; 9) cycle length; and, 10) parsimony.
We identified 28 items related to data issues and broadly classified them into 11 domains: 1) data identification; 2) data modelling; 3) baseline data; 4) treatment effects; 5) risk factors; 6) data incorporation; 7) assessment of uncertainty; 8) methodological; 9) structural; 10) heterogeneity; and, 11) parameter.
We identified 14 items related to consistency (internal and external) and validity (output plausibility and predictive validity). The final five items fell under computer implementation, transparency or funding.
The items are not mutually exclusive, and there is overlap if one takes into account implicit and explicit considerations. Even considering this, the records differed in terms of their comprehensiveness and the areas of model quality they considered. No item was identified by all of the resources, one item appeared in five lists, four items appeared in four lists, three items appeared in 17 lists and the remainder of the items appeared in only one or two lists (Table 1).
Quality of reporting
We identified two papers that addressed reporting practices of modelling studies, the first of which was a systematic review of coronary heart disease policy models [11].
The authors evaluated 75 papers on the basis of whether a sensitivity analysis was carried out, the validity was checked, data quality was reported, illustrative examples were provided, if the model was potentially available to the reader (transparency), and if potential limitations were specified or discussed. This evaluation was based on authors reporting on the specific item in the articles.
Relatively few papers included in the review reported on quality issues: sensitivity analysis and assessment of validity were reported in very few models, 33% provided illustrative examples, working versions of the model were available in 10%, and 19% reported on limitations of their methodology,
The second paper examining the reporting practices of modelling studies looked more specifically at the reporting of calibration methods in 154 cancer simulation models [9]. Data elements abstracted included whether model validation was mentioned (52%) and if a description of the calibration protocol was provided (66%). The authors further characterized calibration protocols by five components. A description of the data used as calibration targets was reported by 95% of the studies and goodness-of-fit metrics were reported in 54% of the studies. However, the search algorithm used for selected alternative parameter values, the criteria for identifying parameter sets that provide an acceptable model fit, and the stopping criteria were not well reported (quantitative values not provided).
Few studies were identified that addressed the quality of reporting of population modelling studies. Overall, with the exception of describing the data used for calibration, there is little consistency in the reporting of items that have been identified as key quality items.
Conclusions
Population modelling studies can fill an important role for policy makers. Their ability to synthesize data from multiple sources and estimate the effects of interventions can be invaluable, especially in areas where primary data collection may be infeasible. However, in order for modelling to gain strength as a tool for health policy, it is critical that key model factors are made transparent so that users of models have a clear understanding of the model and its limitations.
While numerous guidelines exist for developing and evaluating health technology assessment and economic evaluation models, which by extension can be applicable to population modelling studies, there is variation in their comprehensiveness and in the consistency of reporting these methods. There is evidence to suggest that key items are under-reported.
In other areas where reporting guidelines have been developed, there has been a favourable impact on the transparency and accuracy of reporting [12–15]. Population modelling studies may be another area which would benefit from the development of a reporting guideline. Moher and colleagues have outlined the importance of a structured approach to the development of reporting guidelines [6]. This paper provides results from initial steps in this structure approach. Future work should focus on identifying key information related to potential sources of bias in population modelling studies and identifying a multidisciplinary expert panel to steer the guideline development process.
Appendix
Search strategy
-
1.
"Reproducibility of Results"
-
2.
Quality control/
-
3.
((valid$ or reliab$ or quality or accura$) adj2 (result$ or report$ or data)).tw.
-
4.
(good adj1 practice$).tw.
-
5.
Guidelines as Topic/
-
6.
(guideline$ or checklist$).tw.
-
7.
or/1-6
-
8.
(model$ adj3 (stud$ or method$ or process$ or simulation)).tw.
-
9.
(modelling or modeling).tw.
-
10.
8 or 9
-
11.
Research design/
-
12.
Decision Support Techniques/
-
13.
published literature.tw.
-
14.
Research/
-
15.
or/11-14
-
16.
16 7 and 10 and 15
References
Weinstein MC, Toy EL, Sandberg EA, Neumann PJ, Evans JS, Kuntz KM, Graham JD, Hammitt JK: Modeling for health care and other policy decisions: uses, roles, and validity. Value Health. 2001, 4: 348-361. 10.1046/j.1524-4733.2001.45061.x.
Kopec JA, Fines P, Manuel DG, Buckeridge DL, Flanagan WM, Oderkirk J, Abrahamowicz M, Harper S, Sharif B, Okhmatovskaia A, Sayre EC, Rahman MM, Wolfson MC: Validation of population-based disease simulation models: a review of concepts and methods. [Review]. BMC Publ Health. 2010, 10: 710-10.1186/1471-2458-10-710.
Weinstein MC, O’Brien B, Hornberger J, Jackson J, Johannesson M, McCabe C, Luce BR, ISPOR Task Force on Good Research Practices: Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices–Modeling Studies. Value Health. 2003, 6: 9-17. 10.1046/j.1524-4733.2003.00234.x.
Garrison LP: The ISPOR Good Practice Modeling Principles–a sensible approach: be transparent, be reasonable. Value Health. 2003, 6: 6-8. 10.1046/j.1524-4733.2003.00003.x.
Enhancing the Quality and Transparency of Health Research (Equator Network) An introduction to reporting guidelines. http://www.equator-network.org Accessed 2012-03-01.
Moher D, Schulz KF, Simera I, Altman DG: Guidance for developers of health research reporting guidelines. PLoS Medicine / Public Library of Science. 2010, 7: e1000217-
Nuijten MJ, Pronk MH, Brorens MJ, Hekster YA, Lockefeer JH, de Smet PA, Bonsel G, van der Kuy A: Reporting format for economic evaluation. Part II: Focus on modelling studies. Pharmacoeconomics. 1998, 14: 259-268. 10.2165/00019053-199814030-00003.
Drummond M, Manca A, Sculpher M: Increasing the generalizability of economic evaluations: recommendations for the design, analysis, and reporting of studies. Int J Technol Assess Health Care. 2005, 21: 165-171.
Stout NK, Knudsen AB, Kong CY, McMahon PM, Gazelle GS: Calibration methods used in cancer simulation models and suggested reporting guidelines. [Review] [180 refs]. PharmacoEconomics. 2009, 27: 533-545. 10.2165/11314830-000000000-00000.
Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, Woolacoot N, Glanville J: Review of guidelines for good practice in decision-analytic modelling in health technology assessment. [Review] [62 refs]. 2001, Winchester, England: Health Technology Assessment, 8: iii-iiv.
Unal B, Capewell S, Critchley JA: Coronary heart disease policy models: a systematic review. [Review] [51 refs]. BMC Publ Health. 2006, 6: 213-10.1186/1471-2458-6-213.
Smidt N, Rutjes AW, van der Windt DA, Ostelo RW, Bossuyt PM, Reitsma JB, Bouter LM, de Vet HC: The quality of diagnostic accuracy studies since the STARD statement: has it improved?. Neurology. 2006, 67: 792-797. 10.1212/01.wnl.0000238386.41398.30.
Plint AC, Moher D, Morrison A, Schulz K, Altman DG, Hill C, Gaboury I: Does the CONSORT checklist improve the quality of reports of randomised controlled trials? A systematic review. Med J Aust. 2006, 185: 263-267.
Smith BA, Lee HJ, Lee JH, Choi M, Jones DE, Bausell RB, Broome ME: Quality of reporting randomized controlled trials (RCTs) in the nursing literature: application of the consolidated standards of reporting trials (CONSORT). Nurs Outlook. 2008, 56: 31-37. 10.1016/j.outlook.2007.09.002.
Prady SL, Richmond SJ, Morton VM, Macpherson H: A systematic evaluation of the impact of STRICTA and CONSORT recommendations on quality of reporting for acupuncture trials. PLoS One. 2008, 3: e1577-10.1371/journal.pone.0001577. Electronic Resource.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2288/12/168/prepub
Acknowledgments
We thank Sascha Davis, MLIS (Librarian, The Ottawa Hospital) for her assistance with designing the electronic search strategy used in this study.
Funding support: Canadian Institutes of Health Research STAR Emerging Team Grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Concept and design (CB, DM); acquisition of data (CB); analysis and interpretation of data (CB, DM); drafting of the manuscript (CB); critical revision of the manuscript for important intellectual content (CB, DM); and final approval of the version to be published (CB, DM). Both authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Bennett, C., Manuel, D.G. Reporting guidelines for modelling studies. BMC Med Res Methodol 12, 168 (2012). https://doi.org/10.1186/1471-2288-12-168
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2288-12-168